Protective role of triiodothyronine in sepsis‑induced cardiomyopathy through phospholamban downregulation
- PMID: 39821325
- PMCID: PMC11781518
- DOI: 10.3892/ijmm.2025.5488
Protective role of triiodothyronine in sepsis‑induced cardiomyopathy through phospholamban downregulation
Abstract
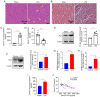
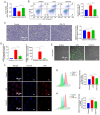
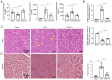
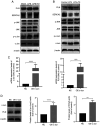
Sepsis is often a cause of mortality in patients admitted to the intensive care unit. Notably, the heart is the organ most susceptible to the impact of sepsis and this condition is referred to as sepsis‑induced cardiomyopathy (SIC). Low triiodothyronine (T3) syndrome frequently occurs in patients with sepsis, and the heart is one of the most important target organs for the action of T3. Phospholamban (PLN) is a key protein associated with Ca2+‑pump‑mediated cardiac diastolic function in the myocardium of mice with SIC, and PLN is negatively regulated by T3. The present study aimed to explore whether T3 can protect cardiac function during sepsis and to investigate the specific molecular mechanism underlying the regulation of PLN by T3. C57BL/6J mice and H9C2 cells were used to establish in vivo and in vitro models, respectively. Myocardial damage was detected via pathological tissue sections, a Cell Counting Kit-8 assay, an apoptosis assay and crystal violet staining. Intracellular calcium levels and reactive oxygen species were detected by Fluo‑4AM and DHE fluorescence. The protein and mRNA expression levels of JNK and c‑Jun were measured by western blotting and reverse transcription‑quantitative PCR to investigate the molecular mechanisms involved. Subsequently, 100 clinical patients were recruited to verify the clinical application value of PLN in SIC. The results revealed a significant negative correlation between PLN and T3 in the animal disease model. Furthermore, the expression levels of genes and proteins in the JNK/c‑Jun signaling pathway and PLN expression levels were decreased, whereas the expression levels of sarcoplasmic reticulum calcium ATPase were increased after T3 treatment. These results indicated that T3 alleviated myocardial injury in SIC by inhibiting PLN expression and its phosphorylation, which may be related to the JNK/c‑Jun signaling pathway. Accordingly, PLN may have clinical diagnostic value in patients with SIC.
Keywords: biomarker; calcium homeostasis; myocardial injury; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Propofol Ameliorates Sepsis-Induced Myocardial Dysfunction via Anti-Apoptotic, Anti-Oxidative Properties, and mTOR Signaling.Discov Med. 2024 Oct;36(189):2088-2097. doi: 10.24976/Discov.Med.202436189.193. Discov Med. 2024. PMID: 39463229
-
Po-Ge-Jiu-Xin decoction alleviate sepsis-induced cardiomyopathy via regulating phosphatase and tensin homolog-induced putative kinase 1 /parkin-mediated mitophagy.J Ethnopharmacol. 2025 Jan 30;337(Pt 3):118952. doi: 10.1016/j.jep.2024.118952. Epub 2024 Oct 18. J Ethnopharmacol. 2025. PMID: 39426573
-
Protective effects of Dioscin against sepsis-induced cardiomyopathy via regulation of toll-like receptor 4/MyD88/p65 signal pathway.Immun Inflamm Dis. 2024 May;12(5):e1229. doi: 10.1002/iid3.1229. Immun Inflamm Dis. 2024. PMID: 38775678 Free PMC article.
-
New developments in the role of ferroptosis in sepsis‑induced cardiomyopathy (Review).Mol Med Rep. 2025 May;31(5):118. doi: 10.3892/mmr.2025.13483. Epub 2025 Mar 7. Mol Med Rep. 2025. PMID: 40052561 Free PMC article. Review.
-
Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions.Cardiovasc Res. 2005 Dec 1;68(3):366-75. doi: 10.1016/j.cardiores.2005.08.010. Epub 2005 Oct 13. Cardiovasc Res. 2005. PMID: 16226237 Review.
References
-
- Mahla RS, Vincent JL, Sakr Y, ICON and SOAP Investigators Sepsis is a global burden to human health: Incidences are underrepresented: Discussion on 'comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP)'. Intensive Care Med. 2018;44:1197–1198. doi: 10.1007/s00134-018-5239-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
