A phase I study of MLN4924 and belinostat in relapsed/refractory acute myeloid leukemia or myelodysplastic syndrome
- PMID: 39821392
- PMCID: PMC11742280
- DOI: 10.1007/s00280-024-04742-9
A phase I study of MLN4924 and belinostat in relapsed/refractory acute myeloid leukemia or myelodysplastic syndrome
Abstract
Purpose: Relapsed and/or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome continue to have a poor prognosis with limited treatment options despite advancements in rational combination and targeted therapies. Belinostat (an HDAC inhibitor) and Pevonedistat (a NEDD8 inhibitor) have each been independently studied in hematologic malignancies and have tolerable safety profiles with limited single-agent activity. Preclinical studies in AML cell lines and primary AML cells show the combination to be highly synergistic, particularly in high-risk phenotypes such as p53 mutant and FLT-3-ITD positive cells. Here, we present the safety, pharmacokinetics and pharmacodynamics of belinostat and pevonedistat in a dose escalation Phase I study in AML and High-Risk MDS.
Methods: Eighteen patients (16 with AML, 2 with MDS) were treated at 5 dose levels (belinostat 800-1000 mg/m2, pevonedistat 20-50 mg/m2). Safety and tolerability were assessed according to protocol defined dose limiting toxicities (DLTs). Correlative pharmacokinetic and pharmacodynamic analyses were performed.
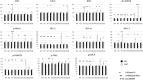
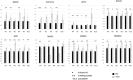
Results: No dose limiting toxicities were noted. Most Grade 3 or 4 toxicities were hematologic in nature. The best response was stable disease in four patients, and complete remission in one patient who qualified as an exceptional responder. Pharmakokinetic studies revealed no association between drug exposure and best response. Pharmacodynamic RT-PCR studies demonstrated post-treatment increases in several proteins, including quantitative increases in the oxidative stress protein NQO1, ferroptosis protein SLC7A11, and GSR, linked to glutathione metabolism and oxidative stress, as did the anti-oxidants SRXN1 and TXNRD1.
Conclusions: Patterns of post-treatment changes in correlative pharmacodynamic parameters may suggest possible mechanistic changes in the DNA damage response, oxidative damage, and ferroptosis pathways. The combination of pevonedistat plus belinosat is safe in an adult relapsed and/or refractory AML/High-Risk MDS population with modest but notable activity in this heavily treated, high risk population. Our findings also raise the possibility that certain extremely poor prognosis AML patients may respond to a regimen combining two targeted agents that have little or no activity when administered individually.
Trial registration: ClinicalTrials.gov ID NCT03772925, first posted 12/12/2018; CTEP Identifier 10246.
Keywords: Acute myeloid leukemia; Belinostat; Myelodysplastic syndrome; Pevonedistat.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval and consent to participate: NCI Central IRB served as the IRB or Record for this study. All patients provided signed informed consent prior to participation in this study.
Figures
References
-
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H et al (2022) Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140(12):1345–1377 - PubMed
-
- Nath R, Reddy V, Kapur A, Gebregergish S, Gurskyte L, Kulakova M et al (2019) Survival of relapsed/refractory acute myeloid leukemia (R/R AML) patients receiving stem cell transplantation (SCT). Biol Blood Marrow Transplant 25(3):S125
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

