Cost-consequence analysis of early vs. delayed natalizumab use in highly active relapsing-remitting multiple sclerosis: a simulation study
- PMID: 39821478
- PMCID: PMC11742466
- DOI: 10.1007/s00415-024-12723-4
Cost-consequence analysis of early vs. delayed natalizumab use in highly active relapsing-remitting multiple sclerosis: a simulation study
Abstract
Background: Natalizumab (NAT) is an established disease-modifying therapy (DMT) for highly active multiple sclerosis (MS). However, its use involves complex decision-making, often leading to initial use of lower efficacy therapies. Recently, the first biosimilar NAT was approved, enabling competitive pricing. This study assessed the societal implications of initiating NAT in various scenarios through a cost-consequence analysis.
Methods: A 10-year Markov model based on the Expanded Disability Status Scale (EDSS) was employed, with 11 health states, annual cycles, and half-cycle correction. The cohort had an initial age of 36 years and 70% females. NAT was compared to common initial therapies (glatiramer acetate, teriflunomide, dimethyl fumarate, and fingolimod). Scenarios included continuous use, early (after 1 year), and delayed (5 years) switch to NAT. Baseline characteristics and probabilities for clinical and economic outcomes were derived from clinical trial data, published literature, and other available sources.
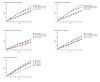
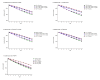
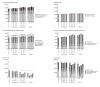
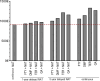
Results: Continuous NAT use resulted in the highest time spent on low EDSS levels, fewer relapses, reduced years of life lost due to disability, and a higher employment rate over a 10-year period. Switching to NAT after 1 year yielded outcomes similar to continuous NAT use. Despite higher DMT costs, disease management costs, including indirect costs and non-DMT direct medical costs, were lower in continuous use and early switch to NAT. Late switching resulted in outcomes most comparable to continuous use of the initial DMT.
Conclusion: Continuous and early switch to NAT resulted in better clinical outcomes and lower societal economic burden compared to delayed NAT initiation, indicating potential long-term cost savings.
Keywords: Cost–consequence; Economic evaluation; Health economics; Markov model; Multiple sclerosis; Treatment strategies.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: HI received speaker honoraria from Roche and financial support for research activities from Teva, Biogen, and Alexion. N-HN is an employee of Hexal AG. K.A. received personal compensation from Novartis, Biogen Idec, Teva, Sanofi, and Roche for consulting services. TZ reports scientific advisory board and/or consulting for Biogen, Roche, Novartis, Celgene, and Merck; compensation for serving on speakers bureaus for Roche, Novartis, Merck, Sanofi, Celgene, and Biogen; and research support from Biogen, Novartis, Merck, and Sanofi. DS and AD have nothing to declare. Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Figures



Similar articles
-
Cost-effectiveness analysis of escalating to natalizumab or switching among immunomodulators in relapsing-remitting multiple sclerosis in Italy.BMC Health Serv Res. 2019 Jun 28;19(1):436. doi: 10.1186/s12913-019-4264-1. BMC Health Serv Res. 2019. PMID: 31253138 Free PMC article.
-
Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England.J Med Econ. 2018 Jul;21(7):676-686. doi: 10.1080/13696998.2018.1461630. Epub 2018 Apr 16. J Med Econ. 2018. PMID: 29618273
-
Cost-effectiveness of alemtuzumab and natalizumab for relapsing-remitting multiple sclerosis treatment in Iran: decision analysis based on an indirect comparison.J Med Econ. 2019 Jan;22(1):71-84. doi: 10.1080/13696998.2018.1543189. Epub 2018 Nov 20. J Med Econ. 2019. PMID: 30380350
-
Comparative effectiveness and cost-effectiveness of natalizumab and fingolimod in rapidly evolving severe relapsing-remitting multiple sclerosis in the United Kingdom.J Med Econ. 2024 Jan-Dec;27(1):109-125. doi: 10.1080/13696998.2023.2293379. Epub 2023 Dec 26. J Med Econ. 2024. PMID: 38085684
-
Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS?Mult Scler Relat Disord. 2017 Oct;17:75-83. doi: 10.1016/j.msard.2017.07.003. Epub 2017 Jul 3. Mult Scler Relat Disord. 2017. PMID: 29055479 Review.
References
-
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391:1622–1636. 10.1016/S0140-6736(18)30481-1 - PubMed
-
- Schriefer D, Haase R, Ness NH, Ziemssen T (2022) Cost of illness in multiple sclerosis by disease characteristics—a review of reviews. Expert Rev Pharmacoecon Outcomes Res 22:177–195. 10.1080/14737167.2022.1987218 - PubMed
-
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910. 10.1056/NEJMoa044397 - PubMed
-
- Butzkueven H, Kappos L, Wiendl H, Trojano M, Spelman T, Chang I, Kasliwal R, Jaitly S, Campbell N, Ho PR et al (2020) Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 91:660–668. 10.1136/jnnp-2019-322326 - PMC - PubMed
-
- Foley JF, Defer G, Ryerson LZ, Cohen JA, Arnold DL, Butzkueven H, Cutter G, Giovannoni G, Killestein J, Wiendl H et al (2022) Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing–remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol 21:608–619. 10.1016/S1474-4422(22)00143-0 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

