Plasma N-terminal tau fragment is an amyloid-dependent biomarker in Alzheimer's disease
- PMID: 39821479
- PMCID: PMC11881634
- DOI: 10.1002/alz.14550
Plasma N-terminal tau fragment is an amyloid-dependent biomarker in Alzheimer's disease
Abstract
Introduction: Novel fluid biomarkers for tracking neurodegeneration specific to Alzheimer's disease (AD) are greatly needed.
Methods: Using two independent well-characterized cohorts (n = 881 in total), we investigated the group differences in plasma N-terminal tau (NT1-tau) fragments across different AD stages and their association with cross-sectional and longitudinal amyloid beta (Aβ) plaques, tau tangles, brain atrophy, and cognitive decline.
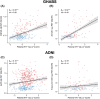
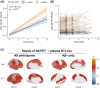
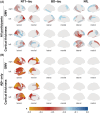
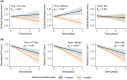
Results: Plasma NT1-tau significantly increased in symptomatic AD and displayed positive associations with Aβ PET (positron emission tomography) and tau PET. Higher baseline NT1-tau levels predicted greater tau PET, with 2- to 10-year intervals and faster longitudinal Aβ PET increases, AD-typical neurodegeneration, and cognitive decline. Plasma NT1-tau showed negative correlations with baseline regional brain volume and thickness, superior to plasma brain-derived tau (BD-tau) and neurofilament light (NfL) in Aβ-positive participants.
Discussion: This study suggests that plasma NT1-tau is an Aβ-dependent biomarker and outperforms BD-tau and NfL in detecting cross-sectional neurodegeneration in the AD continuum.
Highlights: Plasma N-terminal tau (NT1-tau) was specifically increased in the A+/T+ stage. Plasma NT1-tau was positively associated with greater amyloid beta (Aβ) and tau PET (positron emission tomography) accumulations. Higher plasma NT1-tau predicted greater tau burden and faster Aβ increases. Plasma NT1-tau was more related to neurodegeneration than plasma brain-derived tau (BD-tau) and neurofilament light (NfL).
Keywords: Alzheimer's disease; BD‐tau; NT1‐tau; NfL; cognitive decline; neurodegeneration.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors report no competing interests. Author disclosures are available in the Supporting Information.
Figures

Similar articles
-
Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer's disease.Brain. 2020 Dec 1;143(12):3793-3804. doi: 10.1093/brain/awaa342. Brain. 2020. PMID: 33210117 Free PMC article.
-
Elevated plasma neurofilament light was associated with multi-modal neuroimaging features in Alzheimer's disease signature regions and predicted future tau deposition.BMC Neurol. 2024 Jul 6;24(1):236. doi: 10.1186/s12883-024-03728-7. BMC Neurol. 2024. PMID: 38971733 Free PMC article.
-
Associations of hippocampal volumes, brain hypometabolism, and plasma NfL with amyloid, tau, and cognitive decline.Alzheimers Dement. 2025 Feb;21(2):e70005. doi: 10.1002/alz.70005. Alzheimers Dement. 2025. PMID: 39989286 Free PMC article.
-
Predictive Accuracy of Blood-Derived Biomarkers for Amyloid-β Brain Deposition Along with the Alzheimer's Disease Continuum: A Systematic Review.J Alzheimers Dis. 2021;84(1):393-407. doi: 10.3233/JAD-210496. J Alzheimers Dis. 2021. PMID: 34542072
-
Plasma p-tau immunoassays in clinical research for Alzheimer's disease.Alzheimers Dement. 2025 Jan;21(1):e14397. doi: 10.1002/alz.14397. Epub 2024 Dec 3. Alzheimers Dement. 2025. PMID: 39625101 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

