Speech, Language and Non-verbal Communication in CLN2 and CLN3 Batten Disease
- PMID: 39821609
- PMCID: PMC11739554
- DOI: 10.1002/jimd.12838
Speech, Language and Non-verbal Communication in CLN2 and CLN3 Batten Disease
Abstract
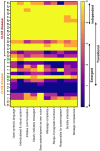
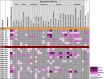
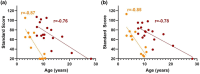
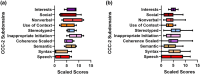
CLN2 and CLN3 diseases, the most common types of Batten disease (also known as neuronal ceroid lipofuscinosis), are childhood dementias associated with progressive loss of speech, language and feeding skills. Here we delineate speech, language, non-verbal communication and feeding phenotypes in 33 individuals (19 females) with a median age of 9.5 years (range 3-28 years); 16 had CLN2 and 17 CLN3 disease; 8/15 (53%) participants with CLN2 and 8/17 (47%) participants with CLN3 disease had speech and language impairments prior to genetic diagnosis. At the time of study all participants, bar one, had language impairments. The remaining participant with typical language was tested at age 3 years, following pre-symptomatic enzyme replacement therapy (ERT) from age 9 months. CLN2 and CLN3 disease had different profiles. For CLN2 disease, all affected individuals showed language impairment with dysarthria; older individuals with classical disease progressively became non-verbal. For CLN3 disease, the presentation was more heterogeneous. Speech impairment was evident early in the disease course, with dysarthria (13/15, 87%), often manifesting as neurogenic stuttering (5/15, 33%). Participants with CLN2 disease had comparable expressive and receptive language skills (p > 0.99), yet participants with CLN3 disease had stronger expressive language than receptive language skills (p = 0.004). Speech, cognitive and language impairment and adaptive behaviour showed progressive decline in both diseases. Individuals with pre-symptomatic ERT or atypical CLN2 disease were less impaired. Challenging behaviours were common in CLN3 (11/17, 65%), but less frequent in CLN2 (4/16, 25%) disease. Individuals with Batten disease require tailored speech therapy incorporating communication partner training utilising environment adaptations and informal communication behaviours.
Keywords: Batten disease; CLN2; CLN3; cerliponase alfa; enzyme replacement therapy; language; neuronal ceroid lipofuscinoses; speech.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Lisa Tilbrook, Michael C. Fahey, Ingrid E. Scheffer and Angela T. Morgan are on the Batten Disease Support and Research Association Australia Medical and Scientific Advisory Board. Angela T. Morganis the director of paediatric/neurodevelopment communication at Redenlab Pty Ltd. and Adam P. Vogel is the Chief Science Officer and Founder of Redenlab Pty Ltd. Ineka T. Whiteman is Head of Research and Medical Affairs, Batten Disease Support, Research and Advocacy Foundation; Principal Scientific Consultant, Beyond Batten Disease Foundation; Head of Research and Medical Affairs, Batten Disease Support and Research Association Australia. Ingrid E. Scheffer is a consultant for Biohaven Pharmaceuticals, Care Beyond Diagnosis, Cerecin Inc., Eisai, Epilepsy Consortium, Longboard Pharmaceuticals, UCB and Zynerba Pharmaceuticals. Ingrid E. Scheffer has received payment honoraria and travel support from Biocodex, BioMarin, Chiesi, Eisai, GlaxoSmithKline, Liva Nova, Nutricia, Stoke Therapeutics, UCB and Zuellig Pharma. Ingrid E. Scheffer is on scientific advisory boards for Bellberry Ltd., BioMarin, Chiesi, Eisai, Encoded Therapeutics, Garvan Institute of Medical Research, Knopp Biosciences, Longboard Pharmaceuticals, UCB and Takeda Pharmaceuticals. Ingrid E. Scheffer is a director at Bellberry Ltd., Australian Academy of Health and Medical Sciences and Australian Council of Learned Academies Ltd. Ingrid E. Scheffer is a trial investigator for Anavex Life Sciences, Cerebral Therapeutics, Cerecin Inc., Cereval Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc., ES‐Therapeutics, GW Pharmaceuticals, Marinus Pharmaceuticals, Neuren Pharmaceuticals, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix and Zynerba Pharmaceuticals. Ingrid E. Scheffer has a patent issued for # Molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 & 2012900190 and PCT/AU2012/001321 (TECH ID:2012–009)
Figures

Similar articles
-
Evidence of the impact of CLN2 and CLN3 Batten disease on families in the United Kingdom.Orphanet J Rare Dis. 2025 May 12;20(1):223. doi: 10.1186/s13023-025-03747-8. Orphanet J Rare Dis. 2025. PMID: 40355884 Free PMC article.
-
Impact of CLN3 Disease on Child Quality of Life and Family Function.Pediatr Neurol. 2025 Sep;170:17-25. doi: 10.1016/j.pediatrneurol.2025.06.008. Epub 2025 Jun 16. Pediatr Neurol. 2025. PMID: 40602048
-
Characterisation of sleep in a mouse model of CLN3 disease revealed sex-specific sleep disturbances.J Sleep Res. 2025 Aug;34(4):e14461. doi: 10.1111/jsr.14461. Epub 2025 Jan 28. J Sleep Res. 2025. PMID: 39873354
-
Parent-mediated communication interventions for improving the communication skills of preschool children with non-progressive motor disorders.Cochrane Database Syst Rev. 2018 Jul 24;7(7):CD012507. doi: 10.1002/14651858.CD012507.pub2. Cochrane Database Syst Rev. 2018. PMID: 30040119 Free PMC article.
-
Interventions for childhood apraxia of speech.Cochrane Database Syst Rev. 2018 May 30;5(5):CD006278. doi: 10.1002/14651858.CD006278.pub3. Cochrane Database Syst Rev. 2018. PMID: 29845607 Free PMC article.
Cited by
-
Understanding speech and language in KIF1A-associated neurological disorder.Eur J Hum Genet. 2025 May 16. doi: 10.1038/s41431-025-01867-0. Online ahead of print. Eur J Hum Genet. 2025. PMID: 40379967
References
-
- University College London , “NCL Disease: Mutation and Patient Database,” accessed March 20, 2024, https://www.ucl.ac.uk/ncl‐disease/mutation‐and‐patient‐database.
-
- Mole S., Williams R., and Goebel H., The Neuronal Ceroid Lipofuscinoses (Batten Disease), vol. 78 (New York: Oxford University Press, 2011).
-
- Nickel M., Simonati A., Jacoby D., et al., “Disease Characteristics and Progression in Patients With Late‐Infantile Neuronal Ceroid Lipofuscinosis Type 2 (CLN2) Disease: An Observational Cohort Study,” Lancet. Child & Adolescent Health 2, no. 8 (2018): 582–590, 10.1016/s2352-4642(18)30179-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

