Multifocal osteochondromatous proliferation and paraneoplastic hematologic dyscrasia in the context of latent Epstein-Barr virus reactivation: a case of oncologic and infectious pathophysiology
- PMID: 39821684
- PMCID: PMC12174209
- DOI: 10.1007/s00256-025-04872-y
Multifocal osteochondromatous proliferation and paraneoplastic hematologic dyscrasia in the context of latent Epstein-Barr virus reactivation: a case of oncologic and infectious pathophysiology
Abstract
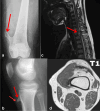
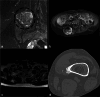
This case report describes a 15-year-old male with multifocal osteochondromatous proliferation and paraneoplastic hematologic dyscrasia, linked to latent Epstein-Barr virus reactivation. Radiographic and advanced imaging revealed widespread skeletal lesions consistent with osteochondromatosis. Hematologic evaluation indicated pancytopenia with dysplastic megakaryocytes and marrow infiltration. Immunohistochemical staining confirmed latent Epstein-Barr virus infection, suggesting its role in the pathogenesis of both the osteochondromatous and hematologic abnormalities. This case highlights the correlation between Epstein-Barr virus reactivation, bone proliferation, and paraneoplastic hematologic processes, which we believe has not yet been reported in the literature, emphasizing the need for a comprehensive diagnostic approach.
Keywords: Bone marrow dysplasia; Epstein-Barr virus (EBV); Multifocal osteochondromatous proliferation; Paraneoplastic hematologic dyscrasia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing of interest: The authors declare no competing interests. Informed consent: Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. The patient has been informed that the information and images will be anonymized, ensuring that no identifying details will be disclosed. This consent process was conducted by ethical guidelines established by the corresponding institution where the study has been reported, ensuring the patient’s autonomy, confidentiality, and rights were fully protected.
Figures

Similar articles
-
MicroRNA changes with macro potential contribute to secondary immunodeficiency in chronic lymphocytic leukemia during epstein barr virus reactivation.Sci Rep. 2025 May 12;15(1):16446. doi: 10.1038/s41598-025-01572-4. Sci Rep. 2025. PMID: 40355604 Free PMC article.
-
Association and Interaction of Epstein-Barr Virus with SARS-CoV-2 Infection-A Review.Viruses. 2025 Jun 26;17(7):903. doi: 10.3390/v17070903. Viruses. 2025. PMID: 40733521 Free PMC article. Review.
-
The PD-1/PD-L1 pathway and Epstein-Barr virus.Eur J Med Res. 2025 Jun 18;30(1):486. doi: 10.1186/s40001-025-02694-1. Eur J Med Res. 2025. PMID: 40533842 Free PMC article. Review.
-
Epstein-Barr virus-positive, primary cutaneous marginal zone lymphoma, with transformation: Case report and review of the literature.Am J Clin Pathol. 2025 Feb 12;163(2):298-312. doi: 10.1093/ajcp/aqae124. Am J Clin Pathol. 2025. PMID: 39290045 Review.
-
T cell-mediated immune surveillance conferred by latent Epstein-Barr virus genes suppresses a broad spectrum of tumor formation through NKG2D-NKG2DL interactions.Front Immunol. 2025 Jun 4;16:1597731. doi: 10.3389/fimmu.2025.1597731. eCollection 2025. Front Immunol. 2025. PMID: 40534857 Free PMC article.
References
-
- Alvarez C, Tredwell S, De Vera M, Hayden M. The genotype-phenotype correlation of hereditary multiple exostoses. Clin Genet. 2006;70(2):122–30. 10.1111/j.1399-0004.2006.00653.x. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

