Pediatric Advance Care Planning and Adolescent Preparedness and Quality of Life: An RCT
- PMID: 39821687
- PMCID: PMC11993244
- DOI: 10.1542/peds.2024-068699
Pediatric Advance Care Planning and Adolescent Preparedness and Quality of Life: An RCT
Abstract
Background and objective: To evaluate the efficacy of Family-Centered Advance Care Planning for Teens With Cancer (FACE-TC) on adolescents' quality of life.
Methods: A clinical trial randomized adolescent-family dyads at a 2:1 ratio to either FACE-TC or control. FACE-TC dyads received 3 weekly 60-minute sessions: Lyon Pediatric Advance Care Planning Survey; Next Steps: Respecting Choices; and Five Wishes. Generalized mixed-effect models evaluated efficacy at 3, 6, and 12 months after intervention measured by FACIT-SP-Ex-V4 (meaning/peace, faith) and PROMIS pediatric (anxiety; depressive symptoms; pain interference, fatigue). Fisher exact tests assessed decisional support and preparedness.
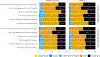
Results: Adolescents (n = 126) were mean age 17 years, 57% female, and 79% white. No significant differences were found between groups for faith or meaning/peace. At 12 months after intervention compared to control, FACE-TC increased anxiety (mean ratio 1.14; CI 1.04-1.25), depressive symptoms (mean ratio 1.12; CI 1.02-1.22), and pain interference (mean ratio 1.10; CI 1.00-1.20), but not at 3 or 6 months. FACE-TC increased fatigue at 3 months (mean ratio 1.13; CI 1.02-1.26), but not at 6 or 12 months. Compared to control, adolescents participating in FACE-TC agreed that "I feel prepared for the future" (76% vs 94%) and "I feel we are now on the same page" (76% vs 94%) at 3 months, but not at 12 months.
Conclusions: There were no significant differences in quality of life between groups until 1 year, except for fatigue. FACE-TC had late effects, increasing adolescents' anxiety, depressive symptoms, and pain interference. Reassessment at 1 year is clinically important.
Copyright © 2025 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
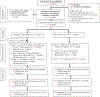

References
-
- Needle JS, Friebert S, Thompkins JD, et al. Effect of the family-centered advance care planning for teens with cancer intervention on sustainability of congruence about end-of-life treatment preferences: a randomized clinical trial. JAMA Netw Open. 2022;5(7): e2220696. PubMed doi: 10.1001/jamanetworkopen.2022.20696 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

