Co-expression of the RPS6KB1 and PDPK1 genes for production of activated p70S6K1 using bac-to-bac baculovirus expression system
- PMID: 39821712
- PMCID: PMC11742003
- DOI: 10.1007/s11033-024-10136-0
Co-expression of the RPS6KB1 and PDPK1 genes for production of activated p70S6K1 using bac-to-bac baculovirus expression system
Abstract
Background: Ribosomal protein S6 kinase 1 (p70S6K1) is a member of the AGC family of serine/threonine kinases which plays a role in various cellular processes, including protein synthesis, cell growth, and survival. Dysregulation of p70S6K1, characterized by its overexpression and/or hyperactivation, has been implicated in numerous human pathologies, particularly in several types of cancer. Therefore, generating active, recombinant p70S6K1 is critical for investigating its role in cancer biology and for developing novel diagnostic or therapeutic approaches.
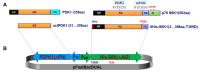
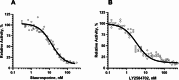
Methods: The baculovirus dual expression system was utilized, enabling the co-expression of two recombinant proteins in infected cells: (a) His-tagged S6K1 with a deletion of the C-terminal autoinhibitory motif and a phosphomimetic mutation at the mTORC1 phosphorylation site (T389D), and (b) untagged PDPK1 lacking the PH domain. The high activity of the purified kinase was confirmed by immunoblotting, as well as by Kinase-Glo and AlphaScreen kinase assays.
Results: Efficient expression of both recombinant proteins was achieved, resulting in highly pure preparations of His-tagged p70S6K1. The high activity of the purified kinase was confirmed through multiple kinase assays, demonstrating significantly higher levels of substrate phosphorylation compared to the tested commercial product.
Conclusion: Here, we report a reliable and efficient methodology for the expression and purification of highly active p70S6K1 (His-actS6K1) in quantity and quality that is suitable for biochemical/biophysical studies and high-throughput enzymatic assays. Our developed methodology offers a rapid and cost-effective approach for producing constitutively active His-actS6K1, which can be utilized in academic research and biotechnology.
Keywords: Baculovirus expression system; Kinase activity; PDPK1; Protein expression; Protein phosphorylation; S6K1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Informed consent: Not applicable. Consent to publish: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Baculovirus-mediated expression, purification, and characterization of a fully activated catalytic kinase domain construct of the 70 kDa 40S ribosomal protein S6 kinase-1 alphaII isoform (S6K1alphaII).Protein Expr Purif. 2008 Mar;58(1):32-41. doi: 10.1016/j.pep.2007.11.003. Epub 2007 Nov 19. Protein Expr Purif. 2008. PMID: 18160308 Free PMC article.
-
Activation of the Stress Response Kinase JNK (c-Jun N-terminal Kinase) Attenuates Insulin Action in Retina through a p70S6K1-dependent Mechanism.J Biol Chem. 2017 Feb 3;292(5):1591-1602. doi: 10.1074/jbc.M116.760868. Epub 2016 Dec 13. J Biol Chem. 2017. PMID: 27965359 Free PMC article.
-
Low-scale phosphoproteome analyses identify the mTOR effector p70 S6 kinase 1 as a specific biomarker of the dual-HER1/HER2 tyrosine kinase inhibitor lapatinib (Tykerb) in human breast carcinoma cells.Ann Oncol. 2008 Jun;19(6):1097-109. doi: 10.1093/annonc/mdm589. Epub 2008 Feb 17. Ann Oncol. 2008. PMID: 18283037
-
Expression, purification, and characterization of a structurally disordered and functional C-terminal autoinhibitory domain (AID) of the 70 kDa 40S ribosomal protein S6 kinase-1 (S6K1).Protein Expr Purif. 2008 Feb;57(2):271-9. doi: 10.1016/j.pep.2007.09.014. Epub 2007 Oct 1. Protein Expr Purif. 2008. PMID: 17980619 Free PMC article.
-
Hydrophobic motif phosphorylation is not required for activation loop phosphorylation of p70 ribosomal protein S6 kinase 1 (S6K1).J Biol Chem. 2011 Jul 1;286(26):23552-8. doi: 10.1074/jbc.M111.258004. Epub 2011 May 11. J Biol Chem. 2011. PMID: 21561857 Free PMC article.
References
-
- Fenton TR, Gout IT (2011) Functions and regulation of the 70 kDa ribosomal S6 kinases. Int J Biochem Cell Biol 43:47–59. 10.1016/j.biocel.2010.09.018 - PubMed
-
- Zaiets IV, Holiar VV, Sivchenko AS, Smialkovska VV, Filonenko VV (2019) p60-S6K1 represents a novel kinase active isoform with the mode of regulation distinct from p70/p85-S6K1 isoforms. UkrBiochemJ 91:17–25. 10.15407/ubj91.04.017
-
- Zaiets IV, Sivchenko AS, Khoruzhenko AI, Savinska LO, Filonenko VV (2018) The Р60-S6K1 isoform of ribosomal protein S6 kinase 1 is a product of alternative mRNA translation. UkrBiochemJ 90:25–35. 10.15407/ubj90.04.025
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

