Red Blood Cell Distribution Width May Predict Drug-Induced Anemia and Prognosis in Patients Affected by Primary/Secondary Myelofibrosis Treated with Ruxolitinib
- PMID: 39821749
- PMCID: PMC11880497
- DOI: 10.1007/s40487-024-00322-2
Red Blood Cell Distribution Width May Predict Drug-Induced Anemia and Prognosis in Patients Affected by Primary/Secondary Myelofibrosis Treated with Ruxolitinib
Abstract
Introduction: Myelofibrosis (MF) is often characterized by a multifactorial anemia determined, in part, by bone marrow (BM) fibrosis, extramedullary erythropoiesis and splenomegaly. Ruxolitinib (RUX) is the first-in-class janus kinase 2 (JAK2) inhibitor approved for treatment of MF, proved to reduce spleen volume and decrease symptom burden. The red cell distribution width (RDW) is the measure of erythrocyte volume variability (anisocytosis). RDW has been recognized as a marker of clinical and subclinical systemic inflammation, and its elevation has also been associated with poor outcome in a wide spectrum of benign disorders and in different types of neoplasms.
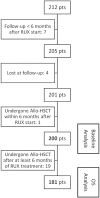
Methods: We retrospectively evaluated RDW in a single-center series of 200 consecutive patients with primary and secondary MF at RUX treatment initiation and examined any possible correlation with adverse MF features or drug-related anemia and any prognostic impact.
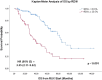
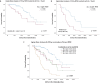
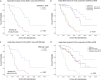
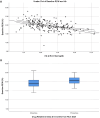
Results: We suggested 20.5% as the optimal cutoff point in RDW values at start of RUX to dichotomize patients in receiver operating characteristic (ROC) analysis for spleen response and for survival. Higher RDW values at RUX start were associated with clinical and laboratory features of an aggressive MF phenotype. Lower spleen response (p < 0.001) and greater odds of drug-related anemia at 3 (p = 0.006) and 6 months (p < 0.001) were also seen in patients with higher RDW. Both increased RDW (considered as a continuous variable) and RDW ≥ 20.5% were associated with shorter overall survival (OS) from RUX initiation in univariate and multivariate analysis: HR 1.25 (95% confidence interval [CI], 1.12-1.40) (p < 0.001) and HR 3.01 (95% CI 1.81-4.99) (p < 0.001), respectively. RDW ≥ 20.5% at RUX start seems to possibly improve patients' sub-stratification along with anemia and conventional prognostic scoring systems.
Conclusions: RDW at RUX start might represent a good indirect measure of MF features and might have prognostic significance for RUX-treated patients affected by MF, aiding in the rapid detection of patients with poor prognosis.
Keywords: Drug-induced anemia; Myelofibrosis; Overall survival (OS); Red blood cell distribution width (RDW); Ruxolitinib (RUX); Spleen response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Massimo Breccia received honoraria from Novartis, Incyte, Pfizer, Abbvie, GSK. Massimo Breccia is an Editorial Board member of Oncology and Therapy. Massimo Breccia was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Maurizio Martelli received honoraria from Roche, Gilead Sciences, Novartis, Abbvie, Incyte, BeiGene, Takeda, and Bristol Myers Squibb/Celgene. Alessandro Laganà, Emilia Scalzulli, Ida Carmosino, and Maria Laura Bisegna declare no conflict of interest. Ethical Approval: This study was a non-interventional, retrospective analysis involving de-identified claims data. All patients signed the informed consent to treatment and to use of their clinical data for scientific purposes. Institutional review board (IRB) approval was not required for this retrospective study as, according to the rules of “Sapienza” University of Rome-Policlinico Umberto I, it is not needed if studies utilize data where formal consent was obtained from patients.
Figures
Similar articles
-
Red cell distribution width and prognosis in myelofibrosis patients treated with ruxolitinib.Ann Hematol. 2024 Aug;103(8):2787-2795. doi: 10.1007/s00277-024-05801-0. Epub 2024 Jun 12. Ann Hematol. 2024. PMID: 38864904
-
Differences in presenting features, outcome and prognostic models in patients with primary myelofibrosis and post-polycythemia vera and/or post-essential thrombocythemia myelofibrosis treated with ruxolitinib. New perspective of the MYSEC-PM in a large multicenter study⁎.Semin Hematol. 2018 Oct;55(4):248-255. doi: 10.1053/j.seminhematol.2018.05.013. Epub 2018 Jun 5. Semin Hematol. 2018. PMID: 30502854
-
Light and shade of ruxolitinib: positive role of early treatment with ruxolitinib and ruxolitinib withdrawal syndrome in patients with myelofibrosis.Expert Rev Hematol. 2022 Jul;15(7):573-581. doi: 10.1080/17474086.2022.2088499. Epub 2022 Jun 14. Expert Rev Hematol. 2022. PMID: 35679520
-
Ruxolitinib: an oral Janus kinase 1 and Janus kinase 2 inhibitor in the management of myelofibrosis.Postgrad Med. 2013 Jan;125(1):128-35. doi: 10.3810/pgm.2013.01.2628. Postgrad Med. 2013. PMID: 23391678 Free PMC article. Review.
-
Classical Philadelphia-negative myeloproliferative neoplasms: focus on mutations and JAK2 inhibitors.Med Oncol. 2018 Aug 3;35(9):119. doi: 10.1007/s12032-018-1187-3. Med Oncol. 2018. PMID: 30074114 Free PMC article. Review.
References
-
- Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol. 2014;89(6):581–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

