Tick salivary cystatin Iristatin limits the virus replication in skin of tick-borne encephalitis virus-infected mice
- PMID: 39821815
- PMCID: PMC11739226
- DOI: 10.1007/s00436-024-08441-5
Tick salivary cystatin Iristatin limits the virus replication in skin of tick-borne encephalitis virus-infected mice
Abstract
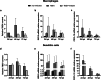
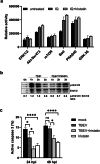
Tick-borne encephalitis virus (TBEV) is flavivirus transmitted to the host via tick saliva which contains various molecules with biological impacts. One of such molecules is Iristatin, a cysteine protease inhibitor from Ixodes ricinus that has been shown to have immunomodulatory properties. To characterize Iristatin in the relation to TBEV, we investigate whether this tick inhibitor has any capacity to influence TBEV infection. Mice were intradermally infected by TBEV with or without Iristatin and the viral multiplication was determined in skin and brain tissues by RT-PCR two and 5 days after infection. The viral RNA was detected in both intervals in skin and increased by time. The application of Iristatin caused a reduction in viral RNA in skin but not in the brain of infected mice 5 days post-infection. Moreover, anti-viral effect of Iristatin on skin was accompanied by a significant decline of interferon-stimulated gene 15 gene expression. The effect of Iristatin on TBEV replication was tested also in vitro in primary macrophages and dendritic cells; however, no changes were observed suggesting no direct interference of Iristatin with virus replication. Still, the Iristatin caused a suppression of Erk1/2 phosphorylation in TBEV-infected dendritic cells and had the anti-apoptotic effect. This is the first report showing that a tick cystatin decreases the viral RNA in the host skin, likely indirectly through creating skin environment that is less supportive for TBEV replication. Assuming, that viral RNA reflects the amount of infectious virus, decline of TBEV in host skin could influence the tick biology or virus transmission during cofeeding.
Keywords: Cystatin; Flavivirus; Tick; Tick-borne encephalitis virus; Virus replication.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All animal experiments were performed in accordance with the Animal Protection Law of the Czech Republic No. 246/1992 Coll. and protocol approved by the Ministry of Education, Youth and Sports of the Czech Republic (protocol no. 14231/2019–2) and the responsible committee of the Faculty of Science, University of South Bohemia. Consent to participate and consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
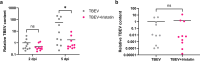

Similar articles
-
The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation.J Virol. 2014 Jun;88(12):6611-22. doi: 10.1128/JVI.03736-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696465 Free PMC article.
-
Saliva of Ixodes ricinus enhances TBE virus replication in dendritic cells by modulation of pro-survival Akt pathway.Virology. 2018 Jan 15;514:98-105. doi: 10.1016/j.virol.2017.11.008. Epub 2017 Nov 20. Virology. 2018. PMID: 29156399
-
An E460D Substitution in the NS5 Protein of Tick-Borne Encephalitis Virus Confers Resistance to the Inhibitor Galidesivir (BCX4430) and Also Attenuates the Virus for Mice.J Virol. 2019 Jul 30;93(16):e00367-19. doi: 10.1128/JVI.00367-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142664 Free PMC article.
-
Tick-Borne Encephalitis Virus: A Structural View.Viruses. 2018 Jun 28;10(7):350. doi: 10.3390/v10070350. Viruses. 2018. PMID: 29958443 Free PMC article. Review.
-
The host cell response to tick-borne encephalitis virus.Biochem Biophys Res Commun. 2017 Oct 28;492(4):533-540. doi: 10.1016/j.bbrc.2017.02.006. Epub 2017 Feb 4. Biochem Biophys Res Commun. 2017. PMID: 28167278 Review.
References
-
- Achazi K, Nitsche A, Patel P, Radonic A, Donoso Mantke O, Niedrig M (2011) Detection and differentiation of tick-borne encephalitis virus subtypes by a reverse transcription quantitative real-time PCR and pyrosequencing. J Virol Methods 171(1):34–39. 10.1016/j.jviromet.2010.09.026 - PubMed
-
- Albarnaz JD et al (2014) MEK/ERK activation plays a decisive role in yellow fever virus replication: implication as an antiviral therapeutic target. Antiviral Res 111:82–92. 10.1016/j.antiviral.2014.09.004 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

