A 10 Year Long-Lived Cellular and Humoral MERS-CoV Immunity Cross-Recognizing the Wild-Type and Variants of SARS-CoV-2: A Potential One-Way MERS-CoV Cross-Protection Toward a Pan-Coronavirus Vaccine
- PMID: 39822038
- PMCID: PMC11740004
- DOI: 10.1002/jmv.70071
A 10 Year Long-Lived Cellular and Humoral MERS-CoV Immunity Cross-Recognizing the Wild-Type and Variants of SARS-CoV-2: A Potential One-Way MERS-CoV Cross-Protection Toward a Pan-Coronavirus Vaccine
Abstract
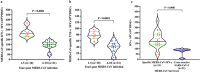
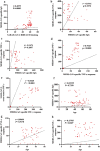
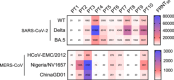
MERS is a respiratory disease caused by MERS-CoV. Multiple outbreaks have been reported, and the virus co-circulates with SARS-CoV-2. The long-term (> 6 years) cellular and humoral immune responses to MERS-CoV and their potential cross-reactivity to SARS-CoV-2 and its variants are unknown. We comprehensively investigated long-lasting MERS-CoV-specific cellular and humoral immunity, and its cross-reactivity against SARS-CoV-2 and its variants, in individuals recovered from MERS-CoV infection 1-10 years prior. Two cohorts of MERS-CoV survivors (31 unvaccinated, 38 COVID-19 vaccinated) were assessed for MERS-CoV IgG, memory CD4+/CD8+ T cells, and neutralizing antibodies against MERS-CoV and SARS-CoV-2 variants. MERS-CoV IgG levels and T cell responses were higher in the 1-5 vs 6-10 year postinfection groups. Vaccinated MERS-CoV survivors had significantly elevated MERS-CoV IgG and neutralization compared to unvaccinated. Both groups demonstrated cross-reactive neutralization of SARS-CoV-2 variants. MERS-CoV survivors vaccinated against SARS-CoV-2 had higher anti-MERS IgG, cellular immunity, and neutralization than unvaccinated survivors. MERS-CoV immune responses can persist for a decade. COVID-19 vaccination boosted humoral and cellular immunity in MERS-CoV survivors, suggesting the benefits of vaccination for this population. These findings have implications for pan-coronavirus vaccine development.
Keywords: BA.5 variant; Delta variant; MERS‐CoV; SARS‐CoV‐2; cellular immunity; cross‐reactivity; humoral immunity; short‐term T cell line.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Cross-Protection against MERS-CoV by Prime-Boost Vaccination Using Viral Spike DNA and Protein.J Virol. 2020 Nov 23;94(24):e01176-20. doi: 10.1128/JVI.01176-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32967955 Free PMC article.
-
Pan-beta-coronavirus subunit vaccine prevents SARS-CoV-2 Omicron, SARS-CoV, and MERS-CoV challenge.J Virol. 2024 Sep 17;98(9):e0037624. doi: 10.1128/jvi.00376-24. Epub 2024 Aug 27. J Virol. 2024. PMID: 39189731 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Immunology and Coronavirus Disease 2019 Clinical Outcomes.Infect Dis Clin North Am. 2025 Jun;39(2):221-232. doi: 10.1016/j.idc.2025.02.001. Epub 2025 Mar 14. Infect Dis Clin North Am. 2025. PMID: 40089444 Review.
-
Durability of immune responses to SARS-CoV-2 infection and vaccination.Semin Immunol. 2024 May;73:101884. doi: 10.1016/j.smim.2024.101884. Epub 2024 Jun 10. Semin Immunol. 2024. PMID: 38861769 Free PMC article. Review.
Cited by
-
Recurrent MERS-CoV Transmission in Saudi Arabia- Renewed Lessons in Healthcare Preparedness and Surveillance.J Epidemiol Glob Health. 2025 Jun 2;15(1):77. doi: 10.1007/s44197-025-00426-6. J Epidemiol Glob Health. 2025. PMID: 40455375 Free PMC article. No abstract available.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

