High Nutritional Conditions Influence Feeding Plasticity in Pristionchus pacificus and Render Worms Non-Predatory
- PMID: 39822045
- PMCID: PMC11788882
- DOI: 10.1002/jez.b.23284
High Nutritional Conditions Influence Feeding Plasticity in Pristionchus pacificus and Render Worms Non-Predatory
Abstract
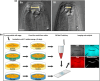
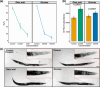
Developmental plasticity, the ability of a genotype to produce different phenotypes in response to environmental conditions, has been subject to intense studies in the last four decades. The self-fertilising nematode Pristionchus pacificus has been developed as a genetic model system for studying developmental plasticity due to its mouth-form polyphenism that results in alternative feeding strategies with a facultative predatory and non-predatory mouth form. Many studies linked molecular aspects of the regulation of mouth-form polyphenism with investigations of its evolutionary and ecological significance. Also, several environmental factors influencing P. pacificus feeding structure expression were identified including temperature, culture condition and population density. However, the nutritional plasticity of the mouth form has never been properly investigated although polyphenisms are known to be influenced by changes in nutritional conditions. For instance, studies in eusocial insects and scarab beetles have provided significant mechanistic insights into the nutritional regulation of polyphenisms but also other forms of plasticity. Here, we study the influence of nutrition on mouth-form polyphenism in P. pacificus through experiments with monosaccharide and fatty acid supplementation. We show that in particular glucose supplementation renders worms non-predatory. Subsequent transcriptomic and mutant analyses indicate that de novo fatty acid synthesis and peroxisomal beta-oxidation pathways play an important role in the mediation of this plastic response. Finally, the analysis of fitness consequences through fecundity counts suggests that non-predatory animals have an advantage over predatory animals grown in the glucose-supplemented condition.
Keywords: Pristionchus pacificus; developmental plasticity; fatty acids; glucose; nutrition; polyphenism.
© 2025 The Author(s). Journal of Experimental Zoology Part B: Molecular and Developmental Evolution published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Alcántar‐Fernández, J. , Navarro R. E., Salazar‐Martínez A. M., Pérez‐Andrade M. E., and Miranda‐Ríos J.. 2018. “ Caenorhabditis elegans Respond to High‐Glucose Diets Through a Network of Stress‐Responsive Transcription Factors.” PLoS One 13: e0199888. 10.1371/journal.pone.0199888. - DOI - PMC - PubMed
-
- Alvarado, S. , Rajakumar R., Abouheif E., and Szyf M.. 2015. “Epigenetic Variation in the Egfr Gene Generates Quantitative Variation in a Complex Trait in Ants.” Nature Communications 6: 6513. - PubMed
-
- Athanasouli, M. , Akduman N., Röseler W., Theam P., and Rödelsperger C.. 2023. “Thousands of Pristionchus pacificus Orphan Genes Were Integrated Into Developmental Networks That Respond to Diverse Environmental Microbiota.” PLoS Genetics 19: e1010832. 10.1371/journal.pgen.1010832. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

