Efficacy of Bilastine, Dextromethorphan, and Phenylephrine Syrup in Patients With Dry Cough: A Phase 3 Randomised Trial
- PMID: 39822435
- PMCID: PMC11735601
- DOI: 10.7759/cureus.75836
Efficacy of Bilastine, Dextromethorphan, and Phenylephrine Syrup in Patients With Dry Cough: A Phase 3 Randomised Trial
Abstract
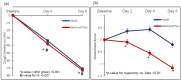
Background Cough in common cold is often associated with rhinorrhoea and nasal congestion, requiring treatment with a cough suppressant, decongestant, and antihistamine. Bilastine is a non-sedating antihistamine, a preferred option over sedating antihistamines. A combination of bilastine, dextromethorphan, and phenylephrine is expected to provide non-sedating treatment for cough associated with a common cold or allergy. Methods In this open-label, randomized, active-controlled, multicentre, clinical trial, adult and adolescent subjects with acute dry cough received bilastine 6.6 mg/dextromethorphan 20 mg/phenylephrine 10 mg syrup (BDP) thrice daily or Alex® syrup (chlorpheniramine maleate 4 mg/dextromethorphan 20 mg/phenylephrine 10 mg) for seven days. The objective of the study was to evaluate the efficacy and safety of a fixed-dose combination syrup of bilastine, dextromethorphan, and phenylephrine in cough relief in patients with cough associated with a common cold or allergy. Results Of 134 randomized subjects, 67 received BDP and the remaining 67 received Alex® syrup. The least-square mean (LSM) standard error (SE) change in cough severity from baseline on day 4 was 37.86 mm (2.012) in the BDP group (p < 0.001), with a mean difference between the two groups of -4.09 mm (95% CI: -9.68, 1.51). The confidence interval was within the non-inferiority (NI) limits of 30 mm, proving non-inferiority to Alex® syrup (p-value for NI < 0.001). The drowsiness score was significantly lower in the BDP group on day 2 (difference -0.46, p = 0.004), day 4 (difference -0.99, p < 0.001), and day 8 (difference -0.97, p < 0.001). No serious or severe adverse event was reported, and adverse events were comparable between the two groups. Conclusion Bilastine/dextromethorphan/phenylephrine combination syrup is efficacious, safe, non-sedating, and non-inferior to Alex® syrup in the treatment of acute dry cough due to a common cold or allergy.
Keywords: allergy; bilastine; common cold; cough; dextromethorphan; phenylephrine.
Copyright © 2024, Kodgule et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Suyash Hospital Institutional Ethics Committee issued approval GPL/CT/2022/001/III. Statement from the ethics committee: We approve the trial to be conducted in its presented form at the following site: Suyash Institute of Medical Science Pvt. Ltd. The above approval is 1 of 10 ethics committee approvals taken corresponding to 10 study sites. The scanned copies of all the IRB approval letters have been sent to the editor by a member of the Cureus Support team owing to a technical issue with uploading figures to the Appendices section (as suggested) as per Request #108307. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Dr Rahul Kodgule, Wen Wu, Amol Pendse, Dr Sheldon Creado, Dr Saiprasad Patil, Dr Hanmant Barkate and Dr Monika Tandon declare(s) employment from Glenmark Pharmaceuticals Limited. The authors mentioned are employees of Glenmark Pharmaceuticals Limited. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Cough management: a practical approach. De Blasio F, Virchow JC, Polverino M, et al. https://pubmed.ncbi.nlm.nih.gov/21985340/ Cough. 2011;7:7. - PMC - PubMed
-
- ERS guidelines on the assessment of cough. Morice AH, Fontana GA, Belvisi MG, et al. Eur Respir J. 2007;29:1256–1276. - PubMed
-
- Treatment of the common cold in children and adults [Internet] Fashner J, Ericson K, Werner S. https://www.aafp.org/pubs/afp/issues/2012/0715/p153.html. Am Fam Physician. 2012;86:153–159. - PubMed
-
- Bilastine: a new antihistamine with an optimal benefit-to-risk ratio for safety during driving. Jáuregui I, Ramaekers JG, Yanai K, Farré M, Redondo E, Valiente R, Labeaga L. https://pubmed.ncbi.nlm.nih.gov/26571227/ Expert Opin Drug Saf. 2016;15:89–98. - PubMed
-
- Use of patient and investigator global impression scales: a review of Food and Drug Administration-Approved Labeling, 2009 to 2019. Gnanasakthy A, Barrett A, Norcross L, D'Alessio D, Romano CD. https://www.sciencedirect.com/science/article/pii/S1098301521001054. Value Health. 2021;24:1016–1023. - PubMed
LinkOut - more resources
Full Text Sources
