Disease characteristics and outcomes of acute myeloid leukemia in germline RUNX1 deficiency (Familial Platelet Disorder with associated Myeloid Malignancy)
- PMID: 39822584
- PMCID: PMC11735945
- DOI: 10.1002/hem3.70057
Disease characteristics and outcomes of acute myeloid leukemia in germline RUNX1 deficiency (Familial Platelet Disorder with associated Myeloid Malignancy)
Abstract
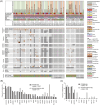
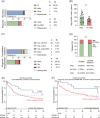
Familial Platelet Disorder with associated Myeloid Malignancy (FPDMM, FPD/AML, RUNX1-FPD), caused by monoallelic deleterious germline RUNX1 variants, is characterized by bleeding diathesis and predisposition for hematologic malignancies, particularly myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Clinical data on FPDMM-associated AML (FPDMM-AML) are limited, complicating evidence-based clinical decision-making. Here, we present retrospective genetic and clinical data of the largest cohort of FPDMM patients reported to date. We describe 159 European patients (from 94 families) of whom 134 were evaluable for the development of malignant disease. Sixty developed a hematologic malignancy (44.8%), most frequently AML (36/134, 26.9%) or MDS (18/134, 13.4%). Somatic alterations of RUNX1 by gene mutation (48%) and chromosome 21 aberrations (14.3%) were the most common somatic genetic aberrations in FPDMM-AML, followed by FLT3-ITD mutations (24.1%). Somatic RUNX1 and FLT3-ITD mutations were not detected in FPDMM-associated MDS, suggesting important contributions to leukemic transformation. Remission-induction chemotherapy resulted in complete remission in 80% of FPDMM-AML patients with a 5-year overall survival (OS) of 50.4%. Survival outcome was non-inferior compared to a large cohort of newly diagnosed adult RUNX1-mutated AML (5-year OS 36.6%, p = 0.5), with relatively infrequent concurrent adverse risk somatic aberrations (ASXL1 mutation, monosomal karyotype, monosomy 5/del 5q) in FPDMM-AML. Collectively, data support the notion that step-wise leukemic evolution in FPDMM is associated with distinct genetic events and indicate that a substantial subset of FPDMM-AML patients achieves prolonged survival with conventional AML treatment, including allogeneic stem cell transplant. These findings are anticipated to inform personalized clinical decision-making in this rare disorder.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Homan CC, King‐Smith SL, Lawrence DM, et al. The RUNX1 database (RUNX1db): establishment of an expert curated RUNX1 registry and genomics database as a public resource for familial platelet disorder with myeloid malignancy. Haematologica. 2021;106(11):3004‐3007. 10.3324/haematol.2021.278762 - DOI - PMC - PubMed
-
- Deuitch N, Broadbridge E, Cunningham L, Liu P. RUNX1 familial platelet disorder with associated myeloid malignancies. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews® [Internet]. University of Washington, Seattle; 2024. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
