Idecabtagene vicleucel or ciltacabtagene autoleucel for relapsed or refractory multiple myeloma: An international multicenter study
- PMID: 39822585
- PMCID: PMC11735948
- DOI: 10.1002/hem3.70070
Idecabtagene vicleucel or ciltacabtagene autoleucel for relapsed or refractory multiple myeloma: An international multicenter study
Abstract
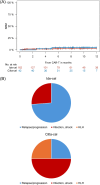
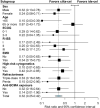
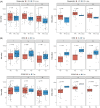
Idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) have revolutionized the treatment of relapsed/refractory multiple myeloma (RRMM), but direct comparisons are lacking. Leveraging an international multicenter RRMM cohort, we compared the outcome of ide-cel (n = 162) versus cilta-cel (n = 42). Co-primary efficacy endpoints of the study were overall response rate (ORR) and progression-free survival (PFS). Co-primary safety endpoints were the incidence of cytokine release syndrome (CRS) and immune-effector cell-associated neurotoxicity syndrome (ICANS). Median turnaround time between apheresis and infusion was 47 days for ide-cel versus 68 days for cilta-cel (p < 0.001). Cilta-cel showed significantly higher ORR (93% vs. 79%; p < 0.001), with complete response at Day 30 of 48% versus 26% (p < 0.001). The 10-month PFS and overall survival (OS) was 82% and 90% for cilta-cel versus 47% and 77% ide-cel (p < 0.001 and p = 0.06), and improved outcome for cilta-cel was confirmed after multivariable adjustment. Incidence of CRS and ICANS appeared similar (81% and 19% for cilta-cel versus 85% and 19% for ide-cel), while 10% and 7% in the cilta-cel group versus 4% and 2% in the ide-cel group showed severe CRS and ICANS grade 3-4, with CRS occurring significantly earlier for ide-cel (median, 2 days vs. 4 days; p < 0.001). Nonrelapse mortality was 5% for cilta-cel versus 3% for ide-cel (p = 0.51). Cilta-cel showed later peak of CAR-T expansion at Day 14 versus Day 7 for ide-cel, while cilta-cel expansion was associated with ICANS. Our study provides real-world evidence that cilta-cel was associated with superior outcomes and distinct cellular dynamics versus ide-cel in triple-class exposed RRMM.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Nico Gagelmann: Consulting or Advisory Role: Stemline Therapeutics, MorphoSys Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Janssen. Maximilian Merz: Honoraria: Janssen, BMS GmbH & Co KG, Amgen, AbbVie, Stemline Therapeutics, Takeda, Sanofi, Pfizer Consulting or Advisory Role: Janssen, BMS GmbH & Co KG, Pfizer, Sanofi Research Funding: Janssen, SpringWorks Therapeutics, Roche/Genentech Travel, Accommodations, Expenses: Janssen, Stemline Therapeutics, Pfizer. Aina Oliver‐Caldés: Travel, Accommodations, Expenses: Janssen. Friedrich Stölzel: Honoraria: Medac, Jazz Pharmaceuticals, Consulting or Advisory Role: Glycostem, Travel, Accommodations, Expenses: SERVIER. Anca‐Maria Albici: Honoraria: AbbVie, Travel, Accommodations, Expenses: SERVIER. Natalie Schub: Honoraria: Janssen Oncology, Consulting or Advisory Role: BMS, Travel, Accommodations, Expenses: Kite/Gilead. Soraya Kharboutli: Honoraria: Bristol Myers Squibb GmbH, Travel, Accommodations, Expenses: Janssen, Bristol Myers Squibb, Sobi, Novartis. Fabian Müller: Honoraria: AstraZeneca, Bristol Myers Squibb/Pfizer, Kite/Gilead, Consulting or Advisory Role: Bristol Myers Squibb/Pfizer, Janssen, Kite/Gilead, Kite/Gilead, Novartis, Miltenyi Biomedicine, Research Funding: Kite/Gilead, Travel, Accommodations, Expenses: SOBI, Janssen. Vladan Vucinic: Honoraria: Janssen, BMS GmbH & Co KG, Gilead Sciences, Amgen, Consulting or Advisory Role: Gilead Sciences, Janssen, BMS GmbH & Co KG, Amgen, Travel, Accommodations, Expenses: Sobi, Janssen, Gilead Sciences, Amgen. Uwe Platzbecker: Honoraria: Celgene/Jazz, AbbVie, Curis, Geron, Janssen, Consulting or Advisory Role: Celgene/Jazz, Novartis, BMS GmbH & Co KG, Research Funding: Amgen (Inst), Janssen (Inst), Novartis (Inst), BerGenBio (Inst), Celgene (Inst), Curis (Inst), Patents, Royalties, Other Intellectual Property: Part of a patent for a TFR‐2 antibody (Rauner et al. Nature Metabolics 2019), Travel, Accommodations, Expenses: Celgene. Francis Ayuk: Honoraria: Bristol Myers Squibb/Celgene, Kite/Gilead, Janssen, Miltenyi Biomedicine, Novartis, Takeda, Mallinckrodt/Therakos, medac pharma, Consulting or Advisory Role: Bristol Myers Squibb/Celgene Research Funding: Mallinckrodt/Therakos. Nicolaus Kröger: Honoraria: Novartis, Celgene (Inst), Sanofi, Jazz Pharmaceuticals (Inst), Kite/Gilead, RIEMSER (Inst), AOP Orphan Pharmaceuticals, BMS GmbH & Co KG, Neovii, Alexion Pharmaceuticals, Takeda, Pierre Fabre Consulting or Advisory Role: Neovii, Sanofi, Jazz Pharmaceuticals, Novartis, Celgene, RIEMSER, Gilead Sciences, Speakers' Bureau: AOP Orphan Pharmaceuticals, Research Funding: Neovii (Inst), Novartis (Inst), Celgene (Inst), Riemser (Inst), Travel, Accommodations, Expenses: Neovii, Novartis, Gilead Sciences, Jazz Pharmaceuticals, Sanofi, Celgene. Carlos Fernández de Larrea: Honoraria: Janssen, BeiGene, Bristol Myers Squibb/Celgene, Pfizer, Amgen, GlaxoSmithKline Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Amgen, Pfizer, Sanofi, BeiGene Research Funding: Janssen (Inst), Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst) Travel, Accommodations, Expenses: Janssen, Amgen, GlaxoSmithKline, Bristol Myers Squibb/Celgene, BeiGene, Pfizer. No other potential conflicts of interest were reported.
Figures


References
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B‐cell maturation antigen‐directed chimeric antigen receptor T‐cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE‐1): a phase 1b/2 open‐label study. Lancet. 2021;398(10297):314‐324. 10.1016/S0140-6736(21)00933-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources
