Rapid (≤25 °C) cycloisomerization of anhydride-tethered triynes to benzynes - origin of a remarkable anhydride linker-induced rate enhancement
- PMID: 39822903
- PMCID: PMC11734507
- DOI: 10.1039/d4sc07232d
Rapid (≤25 °C) cycloisomerization of anhydride-tethered triynes to benzynes - origin of a remarkable anhydride linker-induced rate enhancement
Abstract
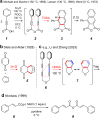
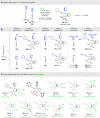
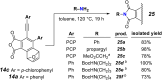
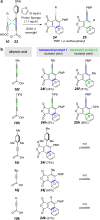
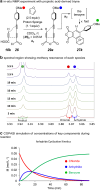
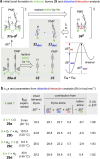
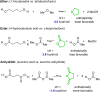
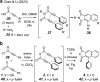
The hexadehydro-Diels-Alder (HDDA) reaction is a cycloisomerization between a conjugated diyne and a tethered diynophile that generates ortho-benzyne derivatives. Considerable fundamental understanding of aryne reactivity has resulted from this body of research. The multi-yne cycloisomerization substrate is typically pre-formed and the (rate-limiting) closure of this diyne/diynophile pair to produce the isomeric benzyne generally requires thermal input, often requiring reaction temperatures of >100 °C and times of 16-48 h to achieve near-full conversion. We report here that diynoic acids can be dimerized and that the resulting substrate, having a 3-atom anhydride linker (i.e., O[double bond, length as m-dash]COC[double bond, length as m-dash]O), then undergoes HDDA cyclization within minutes at or below room temperature. This allows for the novel in situ assembly and cyclization of HDDA benzyne precursors in an operationally simple protocol. Experimental kinetic data along with DFT computations are used to identify the source of this surprisingly huge rate acceleration afforded by the anhydride linker: >107 faster than the analogous multi-yne having, instead, a CH2OCH2 ether linker.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Quaternary Ammonium Ion-Tethered (Ambient-Temperature) HDDA Reactions.J Am Chem Soc. 2022 May 4;144(17):7750-7757. doi: 10.1021/jacs.2c00877. Epub 2022 Apr 20. J Am Chem Soc. 2022. PMID: 35442671 Free PMC article.
-
Hexadehydro-Diels-Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes.Chem Rev. 2021 Feb 24;121(4):2413-2444. doi: 10.1021/acs.chemrev.0c00825. Epub 2021 Jan 25. Chem Rev. 2021. PMID: 33492939 Free PMC article. Review.
-
Mechanistic and Kinetic Factors of ortho-Benzyne Formation in Hexadehydro-Diels-Alder (HDDA) Reactions.Chemistry. 2021 May 26;27(30):7978-7991. doi: 10.1002/chem.202100608. Epub 2021 May 2. Chemistry. 2021. PMID: 33783896 Free PMC article. Review.
-
Silicon as a powerful control element in HDDA chemistry: redirection of innate cyclization preferences, functionalizable tethers, and formal bimolecular HDDA reactions.Chem Sci. 2021 Oct 6;12(41):13902-13908. doi: 10.1039/d1sc04082k. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 34760176 Free PMC article.
-
Photochemical Hexadehydro-Diels-Alder Reaction.J Am Chem Soc. 2017 Jun 28;139(25):8400-8403. doi: 10.1021/jacs.7b03832. Epub 2017 Jun 20. J Am Chem Soc. 2017. PMID: 28548500 Free PMC article.
Cited by
-
Acid-catalyzed, Three-component, Spontaneous Cascades of 1,3-Butadiynyl Propargylic Alcohols as a Route to Phthalan Derivatives.ACS Catal. 2025 Jul 18;15(14):12238-12246. doi: 10.1021/acscatal.5c03571. Epub 2025 Jul 6. ACS Catal. 2025. PMID: 40703645
References
-
- Michael A. Bucher J. E. Chem. Ber. 1895;(28):2511–2512. doi: 10.1002/cber.18950280337. - DOI
-
- Michael A. Bucher J. E. Am. Chem. J. 1898;20:89–127.
-
- Lanser T. Chem. Ber. 1899;(32):2478–2481. doi: 10.1002/cber.189903202195. - DOI
-
- Baddar F. G. El-Assal L. S. J. Chem. Soc. 1948:1267–1270. doi: 10.1039/JR9480001267. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

