Reduction of elevated Gli3 does not alter the progression of autosomal recessive polycystic kidney disease
- PMID: 39823139
- PMCID: PMC11738646
- DOI: 10.14814/phy2.70191
Reduction of elevated Gli3 does not alter the progression of autosomal recessive polycystic kidney disease
Abstract
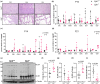
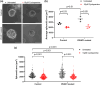
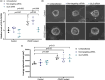
Polycystic kidney diseases (PKD) are genetic disorders which disrupt kidney architecture and function. Autosomal recessive PKD (ARPKD) is a rare form of PKD, caused by mutations in PKHD1, and clinically more severe than the more common autosomal dominant PKD (ADPKD). Prior studies have implicated Hedgehog (Hh) signaling in ADPKD, with increased levels of Hh components in experimental ADPKD and reduced cystogenesis following pharmacological Hh inhibition. In contrast, the role of the Hh pathway in ARPKD is poorly understood. We hypothesized that Hh pathway activity would be elevated during ARPKD pathogenesis, and its modulation may slow disease progression. We utilized Cpk mice which phenocopy ARPKD and generated a PKHD1-mutant spheroid model in human collecting ducts. Significantly elevated levels of the Hh transcriptional effector Gli3 were found in Cpk mice, a finding replicated in PKHD1-mutant spheroids. In Cpk mice, total GLI3 and GLI3 repressor protein levels were also increased. Reduction of increased Gli3 levels via heterozygous genetic deletion in Cpk mice did not affect cyst formation. Additionally, lowering GLI3 transcripts to wildtype levels did not influence PKHD1-mutant spheroid size. Collectively, these data suggest attenuation of elevated Gli3 does not modulate murine and human models of ARPKD.
Keywords: autosomal recessive polycystic kidney disease; cystogenesis; hedgehog signaling; human model; mouse model.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Avner, E. D. , Studnicki, F. E. , Young, M. C. , Sweeney, W. E., Jr. , Piesco, N. P. , Ellis, D. , & Fettermann, G. H. (1987). Congenital murine polycystic kidney disease. Pediatric Nephrology, 1, 587–596. - PubMed
-
- Avner, E. D. , Sweeney, W. E. , Young, M. C. , & Ellis, D. (1988). Congenital murine polycystic kidney disease. II. Pathogenesis of tubular cyst formation. Pediatric Nephrology, 2, 210–218. - PubMed
-
- Bergmann, C. , Senderek, J. , Windelen, E. , Küpper, F. , Middeldorf, I. , Schneider, F. , Dornia, C. , Rudnik‐Schöneborn, S. , Konrad, M. , Schmitt, C. P. , Seeman, T. , Neuhaus, T. J. , Vester, U. , Kirfel, J. , Büttner, R. , & Zerres, K. (2005). Clinical consequences of PKHD1 mutations in 164 patients with autosomal‐recessive polycystic kidney disease (ARPKD). Kidney International, 67, 829–848. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

