The Second Round of a Population-Based Seroprevalence Study of Anti-SARS-CoV-2 Antibodies and COVID-19 Vaccination Assessment in the Republika Srpska, Bosnia and Herzegovina
- PMID: 39823154
- PMCID: PMC11739130
- DOI: 10.1111/irv.70053
The Second Round of a Population-Based Seroprevalence Study of Anti-SARS-CoV-2 Antibodies and COVID-19 Vaccination Assessment in the Republika Srpska, Bosnia and Herzegovina
Abstract
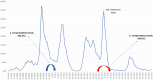
Introduction: The aim of the study was to assess the seroprevalence of SARS-CoV-2 in the Republika Srpska, Bosnia and Herzegovina, after five waves of COVID-19 and 1 year after introduction of vaccination to better understand the true extent of the COVID-19 pandemic in the population of the Republika Srpska and role of vaccination in achieving herd immunity.
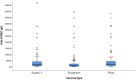
Methods: The population-based study was conducted from December 2021 to February 2022 in a group of 4463 individuals in the Republika Srpska. Total anti-SARS-CoV-2 antibodies were determined in serum specimens using the Wantai total antibody ELISA assay. Quantitative analysis, using Kantaro IgG assays, was performed in subsamples (1273 specimens) to asses and compare levels of IgG among vaccinated, recovered and participants with hybrid immunity. To adjust for age and gender distribution in sample, poststratification method is applied.
Results: The overall cumulative seroprevalence was 94.6% (95% CI = 93.9-95.3). Significantly higher seroprevalence rates were observed among vaccinated 97.8% (95% CI = 97.3-98.4) comparing to unvaccinated participants (89.5%, 95% CI = 88.0-91.0). Seroprevalence increases with the number of received doses. Among various professions, the highest seroprevalence was found in the service industry (98.1%), education (98.0%) and healthcare (96.9%). We found that 2.2% of vaccinated and 3.6% of participants with SARS-CoV-2 positivity during 2021 had no detectable IgG antibodies. Both seroprevalence (98.6%) and antibody titres (1094.4 AU/mL) were significantly higher among people with hybrid immunity.
Conclusion: Our findings reveal a 2.3-fold increase in seroprevalence of SARS-CoV-2 antibodies due to infection and vaccination, comparing to the first study performed 1 year earlier. This study provides better understanding of the SARS-CoV-2 transmission and highlights the important role of the vaccination in achieving the population immunity. Periodically conducted population-based seroprevalence studies are important to understand temporal trends and assess surveillance system performance and public compliance with vaccination policies.
Keywords: Bosnia and Herzegovina; COVID‐19; SARS‐CoV‐2 antibodies; SEROPREV; UNITY; population‐based study; seroprevalence.
© 2025 The Author(s). Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Sero-prevalence of SARS-CoV-2 antibodies in Ethiopia: Results of the National Population Based Survey, 2021.PLoS One. 2025 May 6;20(5):e0313791. doi: 10.1371/journal.pone.0313791. eCollection 2025. PLoS One. 2025. PMID: 40327714 Free PMC article.
-
Estimation of Anti-SARS-CoV-2 IgM/IgG Seroprevalence Among Non-Vaccinated and Vaccinated University Students: A Cross-Sectional Egyptian Study.Viruses. 2025 Mar 6;17(3):378. doi: 10.3390/v17030378. Viruses. 2025. PMID: 40143306 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study.Lancet HIV. 2021 Jun;8(6):e334-e341. doi: 10.1016/S2352-3018(21)00072-2. Epub 2021 Apr 29. Lancet HIV. 2021. PMID: 33933189 Free PMC article.
-
Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: a systematic review and meta-analysis.Int J Infect Dis. 2022 Mar;116:59-67. doi: 10.1016/j.ijid.2021.12.353. Epub 2021 Dec 28. Int J Infect Dis. 2022. PMID: 34968773 Free PMC article.
Cited by
-
A repeated cross-sectional analysis of SARS-CoV-2 seroprevalence in Manila, the Philippines after implementation of the national COVID-19 vaccination program.Trop Med Health. 2025 Jun 16;53(1):81. doi: 10.1186/s41182-025-00767-9. Trop Med Health. 2025. PMID: 40524246 Free PMC article.
References
-
- World Health Organization , “Coronavirus (COVID‐19) Dashboard,” https://covid19.who.int.
-
- Public Health Institute of the Republika Srpska , “COVID‐19: Epidemiological Situation in the Republika Srpska,” cited March 02, 2021, https://www.phi.rs.ba/pdf/sadrzaj/COVID%2019%20%20Epidemioloska16%2001%2....
-
- Ma Q., Liu J., Liu Q., et al., “Global Percentage of Asymptomatic SARS‐CoV‐2 Infections Among the Tested Population and Individuals With Confirmed COVID‐19 Diagnosis: A Systematic Review and Meta‐Analysis,” JAMA Network Open 4, no. 12 (2021): e2137257, 10.1001/jamanetworkopen.2021.37257. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous