Nicotinamide Prevents the Plasticity Impairments and the Cognitive Dysfunction Caused by Bone Fracture in Older Mice
- PMID: 39823270
- PMCID: PMC12363222
- DOI: 10.1093/gerona/glae303
Nicotinamide Prevents the Plasticity Impairments and the Cognitive Dysfunction Caused by Bone Fracture in Older Mice
Abstract
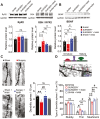
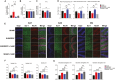
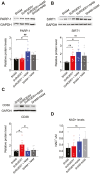
Postoperative delirium (POD), an acute cognitive dysfunction linked to morbidity and mortality, is characterized by memory impairments and disturbances in consciousness, particularly in patients aged 65 and older. Neuroinflammation and NAD+ imbalance are key mechanisms behind POD, leading to synaptic and cognitive deterioration. However, how surgery contributes to POD and neuroinflammation remains unclear, and effective treatments are lacking. Here we used a rodent model of bone fracture to examine the impact of surgery on synaptic plasticity, inflammation, and cognition. Additionally, we explored whether treatment with nicotinamide (NAM), a NAD+ precursor, reduced the neuroinflammation and metabolic imbalance caused by surgery. Female C57BL/6J mice aged 20-22 months underwent tibial fracture surgery and received pre- and post-surgery NAM treatment. Neuroinflammation, synaptic plasticity, and cognition were assessed 72 hours post-surgery via long-term potentiation (LTP) assays, dendritic spine counting, and behavioral tests (open field maze and Y-maze). Tibial fracture surgery decreased LTP, dendritic spine density, and hippocampal-dependent memory function, and increased hippocampal inflammatory markers (IL-1β mRNA, CD38, and SIRT1 protein content); NAM pretreatment prevented these changes. Given surgery's adverse effects on LTP and dendritic spine density, we assessed cellular oxidative state and brain-derived neurotrophic factor (BDNF) protein levels. We found that surgery increased the oxidation of ryanodine receptor calcium channels (cellular redox sensors), and decreased BDNF protein levels; NAM supplementation mitigated both effects and prevented the cognitive decline and synaptic plasticity deficits while reducing inflammation post-surgery by lowering IL-1β and CD38 protein levels. We propose that the CD38 signaling pathway mediates these NAM protective effects.
Keywords: Aging; Animal model; Delirium; Memory; Synaptic plasticity.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Gerontological Society of America.
Conflict of interest statement
The authors declare that there were no commercial or financial relationships present during the research that could be perceived as potential conflicts of interest.
Figures


References
-
- Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primers. 2020;6(1):1–26. https://doi.org/ 10.1038/s41572-020-00223-4 - DOI - PMC - PubMed
-
- Bravo M, Bustos S, Acuña E, et al. Epidemiology of delirium in hospitalized patients in Latin America: a systematic review. Acta Psychiatr Scand. 2023;147(5):420–429. https://doi.org/ 10.1111/acps.13468 - DOI - PubMed
-
- Aung Thein MZ, Pereira JV, Nitchingham A, Caplan GA.. A call to action for delirium research: meta-analysis and regression of delirium associated mortality. BMC Geriatr. 2020;20(1):1–12. https://doi.org/ 10.1186/s12877-020-01723-4 - DOI - PMC - PubMed
-
- Davis D, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(Pt 9):2809–2816. https://doi.org/ 10.1093/brain/aws190 - DOI - PMC - PubMed
-
- González M, Uslar W, Villarroel L, Calderón J, Palma C, Carrasco M.. Hospital costs associated with delirium in older medical patients. Rev Esp Geriatr Gerontol. 2012;47(1):23– 26. https://doi.org/ 10.1016/j.regg.2011.03.005 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

