Viroporin activity is necessary for intercellular calcium signals that contribute to viral pathogenesis
- PMID: 39823322
- PMCID: PMC11740935
- DOI: 10.1126/sciadv.adq8115
Viroporin activity is necessary for intercellular calcium signals that contribute to viral pathogenesis
Abstract
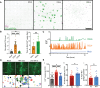
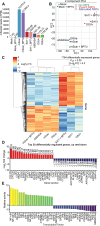
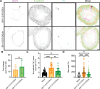
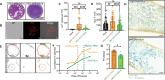
Viruses engage in a variety of processes to subvert host defenses and create an environment amenable to replication. Here, using rotavirus as a prototype, we show that calcium conductance out of the endoplasmic reticulum by the virus encoded ion channel, NSP4, induces intercellular calcium waves that extend beyond the infected cell and contribute to pathogenesis. Viruses that lack the ability to induce this signaling show diminished viral shedding and attenuated disease in a mouse model of rotavirus diarrhea. This implicates nonstructural protein 4 (NSP4) as a virulence factor and provides mechanistic insight into its mode of action. Critically, this signaling induces a transcriptional signature characteristic of interferon-independent innate immune activation, which is not observed in response to a mutant NSP4 that does not conduct calcium. This implicates calcium dysregulation as a means of pathogen recognition, a theme broadly applicable to calcium-altering pathogens beyond rotavirus.
Figures


References
-
- Cohen A. L., Platts-Mills J. A., Nakamura T., Operario D. J., Antoni S., Mwenda J. M., Weldegebriel G., Rey-Benito G., De Oliveira L. H., Ortiz C., Daniels D. S., Videbaek D., Singh S., Njambe E., Sharifuzzaman M., Grabovac V., Nyambat B., Logronio J., Armah G., Dennis F. E., Seheri M. L., Magagula N., Mphahlele J., Fumian T. M., Maciel I. T. A., Gagliardi Leite J. P., Esona M. D., Bowen M. D., Samoilovich E., Semeiko G., Abraham D., Giri S., Praharaj I., Kang G., Thomas S., Bines J., Liu N., Kyu H. H., Doxey M., Rogawski Mcquade E. T., Mcmurry T. L., Liu J., Houpt E. R., Tate J. E., Parashar U. D., Serhan F., Aetiology and incidence of diarrhoea requiring hospitalisation in children under 5 years of age in 28 low-income and middle-income countries: Findings from the Global Pediatric Diarrhea Surveillance network. BMJ Glob. Health 7, 9548 (2022). - PMC - PubMed
-
- King C. K., Glass R., Bresee J. S., Duggan C., Centers for Disease Control and Prevention , Managing acute gastroenteritis among children: Oral rehydration, maintenance, and nutritional therapy. MMWR Recomm. Rep. 52, 1–16 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

