Insights into the origin, hybridisation and adaptation of Candida metapsilosis hybrid pathogens
- PMID: 39823524
- PMCID: PMC11781744
- DOI: 10.1371/journal.ppat.1012864
Insights into the origin, hybridisation and adaptation of Candida metapsilosis hybrid pathogens
Abstract
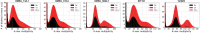
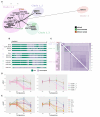
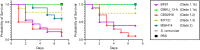
Hybridisation is a source of genetic diversity, can drive adaptation to new niches and has been found to be a frequent event in lineages harbouring pathogenic fungi. However, little is known about the genomic implications of hybridisation nor its impact on pathogenicity-related traits. A common limitation for addressing these questions is the narrow representativity of sequenced genomes, mostly corresponding to strains isolated from infected patients. The opportunistic human pathogen Candida metapsilosis is a hybrid that descends from the crossing between unknown parental lineages. Here, we sequenced the genomes of five new C. metapsilosis isolates, one representing the first African isolate for this species, and four environmental isolates from marine niches. Our comparative genomic analyses, including a total of 29 sequenced strains, shed light on the phylogenetic relationships between C. metapsilosis hybrid isolates and show that environmental strains are closely related to clinical ones and belong to different clades, suggesting multiple independent colonisations. Furthermore, we identify a new diverging clade likely emerging from the same hybridisation event that originated two other previously described hybrid clades. Lastly, we evaluate phenotypes relevant during infection such as drug susceptibility, thermotolerance or virulence. We identify low drug susceptibility phenotypes which we suggest might be driven by loss of heterozygosity events in key genes. We discover that thermotolerance is mainly clade-dependent and find a correlation with the faecal origin of some strains which highlights the adaptive potential of the fungus as commensal.
Copyright: © 2025 del Olmo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Genomic Aftermath of Hybridization in the Opportunistic Pathogen Candida metapsilosis.PLoS Genet. 2015 Oct 30;11(10):e1005626. doi: 10.1371/journal.pgen.1005626. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26517373 Free PMC article.
-
Identification of a novel Candida metapsilosis isolate reveals multiple hybridization events.G3 (Bethesda). 2022 Jan 4;12(1):jkab367. doi: 10.1093/g3journal/jkab367. G3 (Bethesda). 2022. PMID: 34791169 Free PMC article.
-
Genome comparison of Candida orthopsilosis clinical strains reveals the existence of hybrids between two distinct subspecies.Genome Biol Evol. 2014 May;6(5):1069-78. doi: 10.1093/gbe/evu082. Genome Biol Evol. 2014. PMID: 24747362 Free PMC article.
-
Emergence of fungal hybrids - Potential threat to humans.Microb Pathog. 2025 Mar;200:107278. doi: 10.1016/j.micpath.2025.107278. Epub 2025 Jan 11. Microb Pathog. 2025. PMID: 39805347 Review.
-
Candida dubliniensis: ten years on.FEMS Microbiol Lett. 2005 Dec 1;253(1):9-17. doi: 10.1016/j.femsle.2005.09.015. Epub 2005 Sep 26. FEMS Microbiol Lett. 2005. PMID: 16213674 Review.
References
-
- Seyedmousavi S, de Hoog GS, Guillot J, Verweij PE. Emerging and Epizootic Fungal Infections in Animals. Springer; 2018.

