Repeat procedures after pulsed field ablation for atrial fibrillation: MANIFEST-REDO study
- PMID: 39824172
- PMCID: PMC12344414
- DOI: 10.1093/europace/euaf012
Repeat procedures after pulsed field ablation for atrial fibrillation: MANIFEST-REDO study
Abstract
Aims: Initial clinical studies of pulsed field ablation (PFA) to treat atrial fibrillation (AF) indicated a >90% durability rate of pulmonary vein isolation (PVI). However, these studies were largely conducted in single centres and involved a limited number of operators. We aimed to describe the electrophysiological findings and outcomes in patients undergoing repeat ablation after an initial PF ablation for AF.
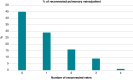
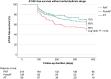
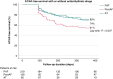
Methods and results: In the MANIFEST-REDO study, we investigated patients who underwent repeat ablation due to clinical recurrence-AF or atrial tachycardia (AT)-following first-ever PVI with a pentaspline PFA catheter (Farawave, Boston Scientific Inc.). At 22 centres, 427 patients (age 64 ± 11 years; 37% female) were included. Of note, the recurrent arrhythmia leading to the repeat ablation was paroxysmal AF (51%), persistent AF (30%), or AT (19%). At the repeat procedure, the PV reconnection rates were 30% (left superior pulmonary vein), 28% (left inferior pulmonary vein), 33% (right superior pulmonary vein), and 32% (right inferior pulmonary vein). In 45% of patients, all PVs were durably isolated at the beginning of the repeat procedure, with the previous use of any imaging or mapping modality being univariately associated with durable PVI. After a post-redo follow-up period of 284 (90-366) days, the primary effectiveness endpoint (freedom from documented AF/AT lasting ≥30 s after 3-month blanking without class I/III antiarrhythmic drugs or symptoms) was achieved in 65% of patients, with significant differences between groups (PAF 65% vs. PersAF 56% vs. AT 76%; P = 0.04). Persistent AF as recurrent arrhythmia after the initial PFA ablation predicted AT/AF recurrence after repeat ablation [hazard ratio 1.241 (95% confidence interval 1.534-1.005); P = 0.045]. The procedural complication rate was 2.8%.
Conclusion: In repeat procedures for AF/AT performed after an index procedure with PFA for AF, PV reconnections are not uncommon. Repeat procedures can be performed safely and with an acceptable subsequent success rate.
Keywords: Atrial fibrillation; Atrial tachycardia; Electroporation; Pulmonary vein isolation; Pulsed field ablation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: D.S. reports receiving consulting fees and speaker honoraria from Abbott, Biosense Webster, Biotronik, and Boston Scientific. M.K.T. reports receiving consulting fees from Biosense Webster, Boston Scientific, and AltaThera, and received speaker honoraria from Sanofi and Medtronic. Y.B. reports receiving research grant from Medtronic and AtriCure and consulting fees from Abbott, Biosense Webster, Boston Scientific, and member of the advisory board for Abbott, Biosense Webster, Boston Scientific, and Medtronic. P.N. reports receiving grant from the Ministry of Health, Czech Republic, DRO (NHH, 00023884). T.R. reports research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, and the sitem-insel support fund; speaker/consulting honoraria or travel support from Abbott/SJM, Bayer, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, Medtronic, and Pfizer-BMS; and support for his institution’s fellowship programme from Abbott/SJM, Biosense Webster, Biotronik, Boston Journal Pre-proof 21 Scientific, and Medtronic. A.M. reports research grant and fees from Farapulse. S.B. reports receiving consulting fees from Medtronic, Boston Scientific, Microport, Zoll, and BMS. A.A. reports receiving consultant fees from Farapulse Inc., Boston Scientific Inc., Galaxy Medical Inc., and Biosense Webster, and performs contracted research for Farapulse Inc., Boston Scientific Inc., Galaxy Medical Inc., and Biosense Webster. J.H. reports receiving speaker fees and grant support from Biosense Webster and Medtronic. M.M. reports receiving speaker fees from Bayer, Biosense Webster, Biotronik, Amomed, AOP Orphan, Boston Scientific, Daiichi Sankyo, and BMS/Pfizer and research grants from Biosense Webster and Abbott. P.S. is an advisory board member of Abbott, Boston Scientific, J&J MedTec, and Medtronic. F.A. reports receiving consulting fees from Boston Scientific, Medtronic, and Microport CRM. S.W. reports receiving grants and personal fees from Abbott, Boston Scientific, and Medtronic, and personal fees from Boehringer Ingelheim, Bristol Myers Squibb, Bayer Vital, Accutus, Daiichi, and Farapulse Inc. T.D. reports receiving speaker honoraria from Galaxy Medical, Abbott, and Biotronik, being a consultant to Farapulse, and serving on a Clinical Events Committee for Boston Scientific. R.T. reports receiving consulting fees from Boston Scientific, Abbott Medical, Biotronik, and Biosense Webster and speaker honorarium from Boston Scientific, Abbott Medical, Biotronik, and Biosense Webster. D.S. reports receiving speaking fees from Pfizer, Bayer, Abbott, Johnson & Johnson, and Medtronic; grant from Abbott, Johnson & Johnson, and Boston Scientific; and consulting fees from Boston Scientific and Johnson & Johnson. R.W. reports receiving honoraria for advisory board activities from Bayer, Boehringer Ingelheim, BMS, Pfizer, Daiichi Sankyo, Boston Scientific, and AtriCure; investigator-initiated funding for research projects (initiated by him) from Abbott, Abiomed, Bristol Myers Squibb, Pfizer, and Boston Scientific; and speaking honoraria from Boston Scientific, Biotronik, Medtronic, Boehringer Ingelheim, Daiichi Sankyo, BMS, Pfizer, Abiomed, Zoll, and Novartis. P.J. reports receiving partial funding from IHU LIRYC ANR-10-IAHU-04 and receiving equity from Farapulse and consulting fees and grant from Boston Scientific. A.R. reports receiving research grant from Farapulse. M.D.L. reports receiving research grant from Farapulse. C.S. reports receiving modest honoraria from Medtronic. K.N. reports speaker’s fees from Farapulse, Inc. M.G. reports grant from Farapulse Inc. and Abbott. A.S. reports receiving lecture and consulting honoraria from Medtronic, Abbott, and Bayer. J.K. reports personal fees from Bayer, Biosense Webster, Boehringer Ingelheim, Medtronic, and Abbott for participation in scientific advisory boards and has received speaker honoraria from Bayer, Biosense Webster, Biotronik, Boehringer Ingelheim, CathVision, Medtronic, Mylan, Pfizer, ProMed, and Abbott. C.-H.H. received travel grants and research grants by Boston Scientific, Lifetech, Biosense Webster, and CardioFocus and speaker’s honoraria from Boston Scientific, Lifetech, Biosense Webster, Bayer, and CardioFocus. He is a consultant of Medtronic, Journal Pre-proof 22 Lifetech, Boston Scientific, Biosense Webster, and CardioFocus. N.D. reports receiving consulting fees from Boston Scientific. V.Y.R. reports receiving consulting fees (and equity—now divested) from Farapulse Inc. and is a consultant for Boston Scientific Inc.; unrelated to this manuscript, he also serves as a consultant for and has equity in Ablacon-Cortex, Acutus Medical, Affera-Medtronic, Anumana, Apama Medical-Boston Scientific, APN Health, Append Medical, Aquaheart, Atacor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, CardioFocus, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, Field Medical, Focused Therapeutics, Heartbeam, HRT, Intershunt, Javelin, Kardium, Laminar Medical, LuxMed, Medlumics, Nuvera-Biosense Webster, Oracle Health, Pulse Biosciences, Restore Medical, Sirona Medical, SoundCath, Volta Medical; unrelated to this work, V.Y.R. has served as a consultant for Abbott, Adagio Medical, AtriAN, Biosense Webster, BioTel Heart, Biotronik, Cairdac, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, Novo Nordisk, Philips; and unrelated to this work, V.Y.R. has equity in Atraverse, DRS Vascular, Manual Surgical Sciences, Newpace, Nyra Medical, Soundcath, Surecor, and Vizaramed. All remaining authors declared no conflict of interest.
Figures

Comment in
-
Pulmonary vein reconnection rates after pulse field ablation: time for a reality check?Europace. 2025 Feb 5;27(2):euaf014. doi: 10.1093/europace/euaf014. Europace. 2025. PMID: 39824176 Free PMC article. No abstract available.
-
Letter on 'Circumferential pulmonary vein isolation (CPVI) with adjunctive linear ablation vs. CPVI alone for long-standing persistent atrial fibrillation: a randomized pilot study'.Europace. 2025 Oct 31;27(11):euaf261. doi: 10.1093/europace/euaf261. Europace. 2025. PMID: 41068972 Free PMC article. No abstract available.
References
-
- Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM et al. 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2024;45:3314–414. - PubMed
-
- Tzeis S, Gerstenfeld EP, Kalman J, Saad EB, Sepehri Shamloo A, Andrade JG et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024;26:euae043.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

