Curcumin mimics of potential chemoprevention with NQO1 induction properties
- PMID: 39824830
- PMCID: PMC11748699
- DOI: 10.1038/s41598-025-85588-w
Curcumin mimics of potential chemoprevention with NQO1 induction properties
Abstract
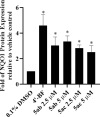
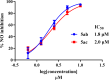
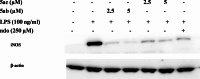
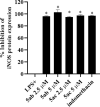
Chemoprevention is one of the accessible strategies for preventing, delaying or reversing cancer processing utilizing chemical intervention of carcinogenesis. NAD(P)H quinone oxidoreductase 1 (NQO1) is a xenobiotic metabolizing cytosolic enzyme/protein with important functional properties towards oxidation stress, supporting its ability in detoxification/chemoprotective role. A set of 3,5-diylidene-4-piperidones (as curcumin mimics) bearing alkyl sulfonyl group were synthesized with potential NQO1 induction properties. Compounds 5ab (R = 2-MeOC6H4, R' = Me) and 5ac (R = 2-MeOC6H4, R' = Et) are the most promising agents synthesized (% induction of NQO1 = 51.562, 45.793) relative to that of 4'-bromoflavone (4'-BF, reference standard) at 10 µM. LPS-induced iNOS production in RAW264.7 macrophages of the most promising agents discovered (5ab and 5ac) displayed concentration-dependent with comparable activities to the reference anti-inflammatory drug indomethacin. Molecular modeling studies (including QSAR, molecular docking and molecular dynamics) were accessed supporting the observed biological profiles.
Keywords: Cancer; Chemoprevention; Curcumin mimic; Molecular modeling; NQO1; Piperidone; iNOS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Acetyl barlerin from Barleria trispinosa induces chemopreventive NQO1 and attenuates LPS-induced inflammation: in vitro and molecular dynamic studies.J Biomol Struct Dyn. 2025 Mar;43(4):2002-2013. doi: 10.1080/07391102.2023.2293272. Epub 2023 Dec 20. J Biomol Struct Dyn. 2025. PMID: 38116740
-
Induction of NQO1 in cancer cells.Methods Enzymol. 2004;382:320-51. doi: 10.1016/S0076-6879(04)82018-4. Methods Enzymol. 2004. PMID: 15047110 Review. No abstract available.
-
GR24, a synthetic analog of Strigolactones, alleviates inflammation and promotes Nrf2 cytoprotective response: In vitro and in silico evidences.Comput Biol Chem. 2018 Oct;76:179-190. doi: 10.1016/j.compbiolchem.2018.07.014. Epub 2018 Jul 18. Comput Biol Chem. 2018. PMID: 30048925
-
Indigofera suffruticosa Mill extracts up-regulate the expression of the π class of glutathione S-transferase and NAD(P)H: quinone oxidoreductase 1 in rat Clone 9 liver cells.Food Chem Toxicol. 2013 Sep;59:610-7. doi: 10.1016/j.fct.2013.06.042. Epub 2013 Jul 3. Food Chem Toxicol. 2013. PMID: 23831193
-
Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes.Methods Enzymol. 2004;382:414-23. doi: 10.1016/S0076-6879(04)82022-6. Methods Enzymol. 2004. PMID: 15047114 Review. No abstract available.
References
-
- Siegel, R. L., Giaquinto, A. N., Jemal, A. & Cancer statistics CA Cancer J. Clin.74, 12–49. (2024). 10.3322/caac.21820 (2024). - PubMed
-
- van Zandwijk, N. Chemoprevention in lung carcinogenesis – an overview. Eur. J. Cancer41, 1990–2002. 10.1016/j.ejca.2005.05.011 (2005). - PubMed
-
- Anisimov, V. N. & Carcinogenesis Aging 20 years after: Escaping horizon. Mech. Ageing Dev.130, 105–121. 10.1016/j.mad.2008.02.004 (2009). - PubMed
-
- Cabrera, M., Mastandrea, I., Otero, G., Cerecetto, H. & González, M. Vivo vivo phase II-enzymes inducers, as potential chemopreventive agents, based on the chalcone and furoxan skeletons. Bioorg. Med. Chem.24, 1665–1674. 10.1016/j.bmc.2016.02.041 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

