Mechanosensation of the heart and gut elicits hypometabolism and vigilance in mice
- PMID: 39824919
- PMCID: PMC12232619
- DOI: 10.1038/s42255-024-01205-6
Mechanosensation of the heart and gut elicits hypometabolism and vigilance in mice
Abstract
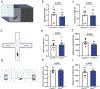
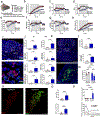
Interoception broadly refers to awareness of one's internal milieu. Although the importance of the body-to-brain communication that underlies interoception is implicit, the vagal afferent signalling and corresponding brain circuits that shape perception of the viscera are not entirely clear. Here, we use mice to parse neural circuits subserving interoception of the heart and gut. We determine that vagal sensory neurons expressing the oxytocin receptor (Oxtr), referred to as NGOxtr, send projections to cardiovascular or gastrointestinal tissues and exhibit molecular and structural features indicative of mechanosensation. Chemogenetic excitation of NGOxtr decreases food and water consumption, and remarkably, produces a torpor-like phenotype characterized by reductions in cardiac output, body temperature and energy expenditure. Chemogenetic excitation of NGOxtr also creates patterns of brain activity associated with augmented hypothalamic-pituitary-adrenal axis activity and behavioural indices of vigilance. Recurrent excitation of NGOxtr suppresses food intake and lowers body mass, indicating that mechanosensation of the heart and gut can exert enduring effects on energy balance. These findings suggest that the sensation of vascular stretch and gastrointestinal distention may have profound effects on whole-body metabolism and, possibly, mental health.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



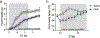



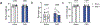



Update of
-
Mechanosensation of the heart and gut elicits hypometabolism and vigilance in mice.bioRxiv [Preprint]. 2023 Jul 1:2023.06.29.547073. doi: 10.1101/2023.06.29.547073. bioRxiv. 2023. Update in: Nat Metab. 2025 Feb;7(2):263-275. doi: 10.1038/s42255-024-01205-6. PMID: 37425814 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R35 HL150750/HL/NHLBI NIH HHS/United States
- R56 AT012142/AT/NCCIH NIH HHS/United States
- 23POST1020034/American Heart Association (American Heart Association, Inc.)
- HL145028/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- AT012142/U.S. Department of Health & Human Services | NIH | National Center for Complementary and Integrative Health (NCCIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

