Oxytocin levels in response to CRH administration in hypopituitarism and hypothalamic damage: a randomized, crossover, placebo-controlled trial
- PMID: 39824923
- PMCID: PMC11742408
- DOI: 10.1038/s41598-025-86566-y
Oxytocin levels in response to CRH administration in hypopituitarism and hypothalamic damage: a randomized, crossover, placebo-controlled trial
Abstract
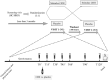
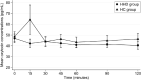
Increasing evidence supports the presence of oxytocin deficiency (OXT-D) in patients with hypopituitarism and hypothalamic damage (HHD), that might be associated with neuropsychological deficits and sexual dysfunction, leading to worse quality of life (QoL). Therefore, identifying a provocative test to diagnose an OXT-D will be important. Corticotropin-releasing hormone (CRH) is a candidate for such a test as it increases oxytocin secretion in animal models. This study aimed to examine the effects of CRH on oxytocin release in HHD compared to healthy controls (HC) and to describe the psychopathology, sexual function and QoL and their associations with oxytocin. This is a single-blind, randomized, placebo-controlled, proof-of-concept study (NCT04902235) with crossover assignment (CRH vs. placebo). Nineteen HHD patients (10 females) and 20 HC (11 females) completed two visits, receiving CRH or placebo in random order and completed validated questionnaires to assess psychopathology, sexual function and QoL. Samples were collected over 120 min to assess oxytocin. Linear mixed-effects regression model evaluated the change in oxytocin after CRH/placebo in HHD vs. HC. CRH administration did not impact oxytocin concentrations across groups over time (p = 0.97). HHD had greater psychopathology (most ps < 0.05), sexual dysfunction (p < 0.03) and worse QoL (p < 0.001) compared to HC, nevertheless, baseline oxytocin concentrations and area under the curve of oxytocin were not significantly associated with psychopathology, sexual function or QoL, neither in HHD or HC. In conclusion, CRH administration does not appear to be a suitable provocative test for diagnosing OXT-D in HHD. Identifying a reliable diagnostic test for OXT-D remains crucial. Alternative provocative tests or biomarkers should be explored.
Keywords: Arginine-vasopressin deficiency; Corticotropin-releasing hormone; Hypopituitarism; Hypothalamic damage; Oxytocin; Pituitary disease.
© 2025. The Author(s).
Conflict of interest statement
Declaration. Competing interests: EAL receives grant support and research study drug from Tonix Pharmaceuticals and receives royalties from UpToDate. EAL and/or immediate family member holds stock in Thermo Fisher Scientific, Zoetis, Danaher Corporation, Intuitive Surgical. EAL is an inventor on US provisional patent application no. 63/467,980 (Oxytocin-based therapeutics to improve cognitive control in individuals with attention deficit hyperactivity disorder). No other conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

