Defective homologous recombination and genomic instability predict increased responsiveness to carbon ion radiotherapy in pancreatic cancer
- PMID: 39824957
- PMCID: PMC11742413
- DOI: 10.1038/s41698-025-00800-4
Defective homologous recombination and genomic instability predict increased responsiveness to carbon ion radiotherapy in pancreatic cancer
Abstract
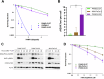
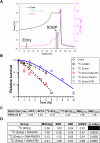
Pancreatic ductal adenocarcinoma (PDAC) is notably resistant to conventional chemotherapy and radiation treatment. However, clinical trials indicate that carbon ion radiotherapy (CIRT) with concurrent gemcitabine is effective for unresectable locally advanced PDAC. This study aimed to identify patient characteristics predictive of CIRT response. We utilized a panel of human PDAC cell lines with diverse genetic profiles to determine their sensitivity to CIRT compared to γ-rays, assessing relative biological effectiveness (RBE) at 10% survival, which ranged from 1.96 to 3.04. Increased radiosensitivity was linked to impaired DNA double-strand break (DSB) repair, particularly in cell lines with deficiencies in the homologous recombination (HR) repair pathway and/or elevated genomic instability from replication stress. Furthermore, pretreatment with the HR inhibitor B02 significantly enhanced CIRT sensitivity in a radioresistant PDAC cell line when irradiated in the spread-out Bragg peak but not at the entry position of the beam. These findings suggest that PDAC tumors with HR pathway mutations or high replication stress are more likely to benefit from CIRT while minimizing normal tissue toxicity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin.73, 17–48 (2023). - PubMed
-
- Kolbeinsson, H. M., Chandana, S., Wright, G. P. & Chung, M. Pancreatic cancer: a review of current treatment and novel therapies. J. Invest Surg.36, 2129884 (2023). - PubMed
-
- Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med.364, 1817–1825 (2011). - PubMed
-
- Conroy, T. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med.379, 2395–2406 (2018). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

