Biochemical failure-free survival of 18F-rhPSMA-7 and 18F-flotufolastat PET-guided salvage radiotherapy for patients with recurrent prostate cancer
- PMID: 39824988
- PMCID: PMC11748678
- DOI: 10.1038/s41598-024-83074-3
Biochemical failure-free survival of 18F-rhPSMA-7 and 18F-flotufolastat PET-guided salvage radiotherapy for patients with recurrent prostate cancer
Abstract
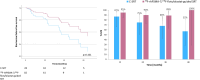
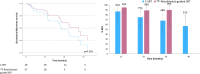
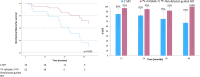
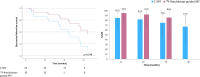
Prostate-specific membrane antigen (PSMA)-targeted positron emission tomography (PET) has improved localization of prostate cancer (PC) lesions in biochemical recurrence (BCR) for salvage radiotherapy (SRT). We conducted a retrospective review of patients undergoing 18F-rhPSMA-7 or 18F-flotufolastat (18F-rhPSMA-7.3)-PET-guided SRT compared with conventional-SRT (C-SRT) without PET. We evaluated biochemical failure-free survival (bFS) and overall rates of bFS in 110 evaluable patients with recurrent PC after radical prostatectomy who received SRT. 82 patients received 18F-rhPSMA-7/18F-flotufolastat-PET-guided SRT and 28 received C-SRT. Median bFS for patients with 18F-rhPSMA-7/18F-flotufolastat-PET-guided SRT was not reached while median bFS was 45.6 months for patients with C-SRT (p = 0.101). %bFS were 95% (52/55) vs 87% (20/23), 90% (27/30) vs 75% (15/20), 89% (16/18) vs 68% (13/19) and 100% (3/3) vs 57% (8/14) for PET-guided vs C-SRT at 12, 24, 36, and 48 months, respectively. Among patients treated in the prostate bed only, median bFS was not reached for PSMA-PET-guided SRT (n = 52) vs 55.1 months in the C-SRT group (n = 25; p = 0.063). %bFS was greater for PSMA-PET-guided SRT than C-SRT at all evaluated timepoints. 18F-rhPSMA-7/18F-flotufolastat-guided SRT yielded favorable disease outcomes. Although statistical significance was not reached, likely due to the limited sample size in this preliminary analysis, our data illustrate potential for 18F-flotufolastat-PET-guided SRT.
Keywords: 18F-flotufolastat; 18F-rhPSMA-7; PSMA-PET; Prostate cancer; Salvage radiotherapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Matthias Eiber reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker) and Janssen Pharmaceuticals (consultant, speakers bureau), Parexel (image review) and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. He and other inventors are entitled to royalties on sales of POSLUMA®. Isabel Rauscher reports research funding and travel support from Blue Earth Diagnostics. All other authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures
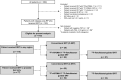
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

