The Role of SIRT1-BDNF Signaling Pathway in Fluoride-Induced Toxicity for Glial BV-2 Cells
- PMID: 39825065
- PMCID: PMC12316830
- DOI: 10.1007/s12011-024-04503-y
The Role of SIRT1-BDNF Signaling Pathway in Fluoride-Induced Toxicity for Glial BV-2 Cells
Abstract
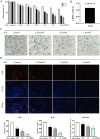
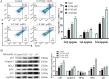
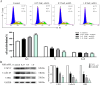
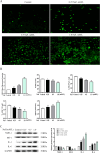
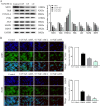
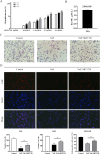
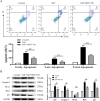
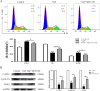
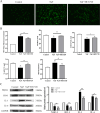
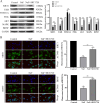
Chronic fluorosis is often accompanied by neurological symptoms, leading to attention, memory and learning ability decline and causing tension, anxiety, depression, and other mental symptoms. In the present study, we analyzed the molecular mechanisms of SIRT1-BDNF regulation of PI3K-AKT, MAPK, and FOXO1A in F-treated BV2 cells. The cytotoxic effect of sodium fluoride (NaF) on BV2 cells was assessed using Cell Counting Kit-8 (CCK-8), crystal violet, and 5-ethynyl-2'-deoxyuridine (EdU) staining. Cell cycle progression and apoptosis were evaluated through flow cytometry and western blotting. Reactive oxygen species (ROS) levels, oxidative stress, and inflammatory markers were measured by ROS staining, microplate reader assays, and western blotting. The role of SIRT1 in fluoride-induced toxicity for glial cells was determined using the SIRT1 activator SRT1720. The experiments demonstrated that NaF was toxic to BV2 cells, inhibited their proliferative ability, halted their cell cycle progression, triggered cellular apoptosis, promoted cellular oxidative stress (detected by ROS, SOD, MDA, GSH-Px, T-AOC) and associated protein NQO-1 and HO-1, and elevated inflammatory mediator associated protein IL-1and IL-6 expression). The fluoride-exposed groups had reduced SIRT1, BDNF, TrkB, PI3K, AKT, and MAPK protein expression levels, and increased FOXO1A protein expression. SRT1720 mitigated the harmful effects of NaF, stimulated cell proliferation and cell cycle progression, decreased apoptosis, reduced oxidative stress and inflammatory factors, elevated SIRT1, BDNF, TrkB, PI3K, AKT, and MAPK protein levels, and suppressed FOXO1A protein expression. The results indicate that NaF potentially harms glial cells by suppressing SIRT1 activation, and SIRT1 significantly mitigated the damage. Furthermore, the SIRT1 signaling pathway might regulate the nerve damage caused by fluoride poisoning and may be a protective factor in treating fluoride-induced brain injury.
Keywords: Fluoride; Inflammation Factors; Nerve Damage; Oxidative Stress; SIRT1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent to Participate: The consent of all authors to participate has been fully confirmed. Consent for Publication: All authors have agreed to submit this paper for publication. Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Clinical Trial Number: Not applicable.
Figures
References
-
- D’Hollander W, Voogt P, Coen W et al (2010) Perfluorinated substances in human food and other sources of human exposure. Rev Environ Contam Toxicol 208:179–215 - PubMed
-
- Zhou J, Sun D, Wei W (2023) Necessity to Pay Attention to the Effects of Low Fluoride on Human Health: an Overview of Skeletal and Non-skeletal Damages in Epidemiologic Investigations and Laboratory Studies. Biol Trace Elem Res 201:1627–1638 - PubMed
MeSH terms
Substances
Grants and funding
- QianKeHe Support [2022]181/Natural Science Foundation of Guizhou Province
- QianKeHe Support ZK[2021]357/Natural Science Foundation of Guizhou Province
- Qiankehe Cooperation Platform talents [2021] Postdoctoral Station 007/Natural Science Foundation of Guizhou Province
- 82060079/National Natural Science Foundation of China
- GuiKeHe XueShuXinMiao [2023]07/Guizhou University of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

