Epicardial adipose tissue, cardiac damage, and mortality in patients undergoing TAVR for aortic stenosis
- PMID: 39825067
- PMCID: PMC11811257
- DOI: 10.1007/s10554-024-03307-4
Epicardial adipose tissue, cardiac damage, and mortality in patients undergoing TAVR for aortic stenosis
Abstract
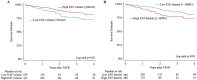
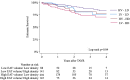
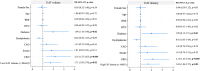
Computed tomography (CT)-derived Epicardial Adipose Tissue (EAT) is linked to cardiovascular disease outcomes. However, its role in patients undergoing Transcatheter Aortic Valve Replacement (TAVR) and the interplay with aortic stenosis (AS) cardiac damage (CD) remains unexplored. We aim to investigate the relationship between EAT characteristics, AS CD, and all-cause mortality. We retrospectively included consecutive patients who underwent CT-TAVR followed by TAVR. EAT volume and density were estimated using a deep-learning platform and CD was assessed using echocardiography. Patients were classified according to low/high EAT volume and density. All-cause mortality at 4 years was compared using Kaplan-Meier and Cox regression analyses. A total of 666 patients (median age 81 [74-86] years; 54% female) were included. After a median follow-up of 1.28 (IQR 0.53-2.57) years, 11.7% (n = 77) of patients died. The EAT volume (p = 0.017) decreased, and density increased (p < 0.001) with worsening AS CD. Patients with low EAT volume (< 49cm3) and high density (≥-86 HU) had higher all-cause mortality (log-rank p = 0.02 and p = 0.01, respectively), even when adjusted for age, sex, and clinical characteristics (HR 1.71, p = 0.02 and HR 1.73, p = 0.03, respectively). When CD was added to the model, low EAT volume (HR 1.67 p = 0.03) and CD stages 3 and 4 (HR 3.14, p = 0.03) remained associated with all-cause mortality. In patients with AS undergoing TAVR, CT-derived low EAT volume, and high density were independently associated with increased 4-year mortality and worse CD stage. Only EAT volume remained associated when adjusted for CD.
Keywords: Aortic stenosis; CCTA; Cardiac damage; Epicardial adipose tissue; TAVI; TAVR.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This is an observational study. The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the a priori approval by the Institution’s Human Research Committee. The study was approved by our Office of Human Research Affairs at Albert Einstein College of Medicine and consent was waived due to study design. Disclosures: Annalisa Filtz, Daniel Lorenzatti, and Leandro Slipczuk are supported by institutional grants from Amgen and Philips. Damini Dey received grant support from the National Heart, Lung, and Blood Institute, software royalties from Cedars-Sinai Medical Center, and holds a patent (US8885905B2 in USA and WO patent WO2011069120A1, Method and System for Plaque Characterization). Others have nothing to declare. Competing interests: Damini Dey received grant support from the National Heart, Lung, and Blood Institute, software royalties from Cedars-Sinai Medical Center, and holds a patent (US8885905B2 in USA and WO patent WO2011069120A1, Method and System for Plaque Characterization). Others have nothing to declare.
Figures


References
-
- Otto CM, Nishimura RA, Bonow RO et al (2021) 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 143(5):e35–e71. 10.1161/CIR.0000000000000932 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

