mtSTAT3 suppresses rheumatoid arthritis by regulating Th17 and synovial fibroblast inflammatory cell death with IL-17-mediated autophagy dysfunction
- PMID: 39825104
- PMCID: PMC11799179
- DOI: 10.1038/s12276-024-01376-y
mtSTAT3 suppresses rheumatoid arthritis by regulating Th17 and synovial fibroblast inflammatory cell death with IL-17-mediated autophagy dysfunction
Abstract
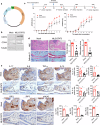
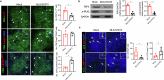
Th17 cells are activated by STAT3 factors in the nucleus, and these factors are correlated with the pathologic progression of rheumatoid arthritis (RA). Recent studies have demonstrated the presence of STAT3 in mitochondria, but its function is unclear. We investigated the novel role of mitochondrial STAT3 (mitoSTAT3) in Th17 cells and fibroblast-like synoviocytes (FLSs) and analyzed the correlation of mitoSTAT3 with RA. We used a collagen-induced arthritis (CIA) mouse model to determine the effect of mitochondrial STAT3. We observed changes in the RA mouse model via the use of a mitochondrial STAT3-inducing vector and inhibitor. We observed the accumulation of abnormal autophagosomes, increased inflammatory cell death signaling, and decreased mitoSTAT3 activity in FLSs from both patients with RA and patients with IL-17-treated FLSs. We first discovered that IL-17 increased the accumulation of abnormal autophagosomes and the expression of inflammatory cell death factors in synovial fibroblasts and decreased mitoSTAT3 activation. In a mouse model of CIA, arthritis and joint inflammation were decreased by injection vectors that induced mitoSTAT3 overexpression. The abnormal accumulation of autophagosomes and the expression of inflammatory cell death factors were also decreased in these mice. In mouse and human immune cells, ZnSO4, an inducer of mitochondrial STAT3, decreases the production of reactive oxygen species, the IL-17 concentration, and differentiation into Th17 cells. However, mitoSTAT3 blockade accelerated the development of arthritis, inflammatory cell death, and abnormal autophagosome/autophagolysosome formation. Therefore, this study suggests a novel inhibitory mechanism of RA using mitoSTAT3 via the regulation of autophagy, Th17 differentiation, and inflammatory cell death.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors have no financial conflicts of interest. All the authors agree with the submission. This work has not been published or submitted for publication elsewhere, either completely or in part or in another form or language. If material has been reproduced from another source, the authors have authorization from the copyright holder (usually the Publisher) to use it and have included this authorization with their submission. Experiments using animal or human materials were approved by national or local authorities.
Figures





References
-
- Yang, C. et al. Altered CD4+ T cell and cytokine levels in peripheral blood and skin samples from systemic sclerosis patients and IL-35 in CD4+ T cell growth. Rheumatology61, 794–805, 10.1093/rheumatology/keab359 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

