Dynamical constraints on neural population activity
- PMID: 39825141
- PMCID: PMC11802451
- DOI: 10.1038/s41593-024-01845-7
Dynamical constraints on neural population activity
Abstract
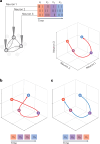
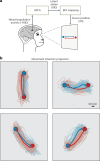
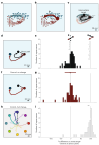
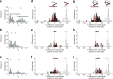
The manner in which neural activity unfolds over time is thought to be central to sensory, motor and cognitive functions in the brain. Network models have long posited that the brain's computations involve time courses of activity that are shaped by the underlying network. A prediction from this view is that the activity time courses should be difficult to violate. We leveraged a brain-computer interface to challenge monkeys to violate the naturally occurring time courses of neural population activity that we observed in the motor cortex. This included challenging animals to traverse the natural time course of neural activity in a time-reversed manner. Animals were unable to violate the natural time courses of neural activity when directly challenged to do so. These results provide empirical support for the view that activity time courses observed in the brain indeed reflect the underlying network-level computational mechanisms that they are believed to implement.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









Update of
-
Dynamical constraints on neural population activity.bioRxiv [Preprint]. 2024 Jan 3:2024.01.03.573543. doi: 10.1101/2024.01.03.573543. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Feb;28(2):383-393. doi: 10.1038/s41593-024-01845-7. PMID: 38260549 Free PMC article. Updated. Preprint.
References
-
- Mazor, O. & Laurent, G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron48, 661–673 (2005). - PubMed
-
- Kim, S. S., Rouault, H., Druckmann, S. & Jayaraman, V. Ring attractor dynamics in the Drosophila central brain. Science356, 849–853 (2017). - PubMed
-
- Chaudhuri, R., Gerçek, B., Pandey, B., Peyrache, A. & Fiete, I. The intrinsic attractor manifold and population dynamics of a canonical cognitive circuit across waking and sleep. Nat. Neurosci.22, 1512–1520 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

