Tucatinib and trastuzumab in HER2-mutated metastatic breast cancer: a phase 2 basket trial
- PMID: 39825152
- PMCID: PMC11922774
- DOI: 10.1038/s41591-024-03462-0
Tucatinib and trastuzumab in HER2-mutated metastatic breast cancer: a phase 2 basket trial
Abstract
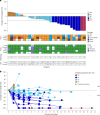
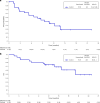
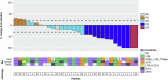
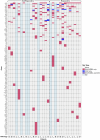
Human epidermal growth factor receptor 2 (HER2, also known as ERBB2) signaling promotes cell growth and differentiation, and is overexpressed in several tumor types, including breast, gastric and colorectal cancer. HER2-targeted therapies have shown clinical activity against these tumor types, resulting in regulatory approvals. However, the efficacy of HER2 therapies in tumors with HER2 mutations has not been widely investigated. SGNTUC-019 is an open-label, phase 2 basket study evaluating tucatinib, a HER2-targeted tyrosine kinase inhibitor, in combination with trastuzumab in patients with HER2-altered solid tumors. The study included a cohort of 31 heavily pretreated female patients with HER2-mutated metastatic breast cancer who were also HER2 negative per local testing. Hormone receptor (HR)-positive patients also received fulvestrant. The overall response rate (primary endpoint) was 41.9% (90% confidence interval (CI): 26.9-58.2). Secondary endpoints of duration of response and progression-free survival were 12.6 months (90% CI: 4.7 to not estimable) and 9.5 months (90% CI: 5.4-13.8), respectively. No new safety signals were detected. Responses were observed across various HER2 mutations, including mutations in the tyrosine kinase and extracellular domains. The chemotherapy-free regimen of tucatinib and trastuzumab showed clinically meaningful antitumor activity with durable responses and favorable tolerability in heavily pretreated patients with HER2 mutations. These data support further investigation of HER2-targeted therapies in this patient population. ClinicalTrials.gov registration: NCT04579380 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.F.C.O. reports research funding from Pfizer and Roche; honoraria for presentations from AstraZeneca, Esai, Gilead, Lilly, Pfizer, Roche and Seagen; advisory board fees from AstraZeneca, Pfizer, Roche and Seagen; and conference support from AstraZeneca, Lilly, Novartis and Roche. G.C. reports consulting or advisory roles with AstraZeneca, Celucity, Daiichi Sankyo, Ellipsis, Exact Sciences, Gilead, Lilly, MBS, Menarini, Merck, Pfizer, Roche and Veracyte; institutional research funding from Astellas, AstraZeneca, Blueprint Medicine, BMS, Daiichi Sankyo, Kymab, Merck, Novartis, Philogen, Relay Therapeutics, Roche and Sanofi; speaker fees from AstraZeneca, Daiichi Sankyo, Novartis, Pfizer and Roche; and writing engagement with Pfizer. N.M. reports grants or contracts to the institution from AstraZeneca, Boehringer Ingelheim, Incyte, MSD, Novartis, Ono Pharmaceutical, Pfizer and Seagen; payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from AstraZeneca, FujiFilm Toyama Chemical, Miyarisan Pharmaceutical, Novartis, Taiho Pharmaceutical and Yakult Honsha; and data safety monitoring or advisory board roles with AstraZeneca and Boehringer Ingelheim. D.-Y.O. reports research funding from Array BioPharma, AstraZeneca, BeiGene, Eli Lilly, Handok, MSD, Novartis and Servier, and consulting or advisory roles with Arcus Biosciences, ASLAN Pharmaceuticals, AstraZeneca, Basilea, Bayer, BeiGene, Celgene, Genentech/Roche, Halozyme, IQVIA, Merck Serono, MSD Oncology, Novartis, Taiho Pharmaceutical, Turning Point Therapeutics, Yuhan and Zymeworks. H.S. reports institutional research funding from Amgen; consulting fees from AstraZeneca, Celgene, Eisai, Novartis, PUMA, Seattle Genetics, Sanofi and Sermonix; advisory board roles with AstraZeneca, Eisai, Eli Lilly, Novartis and PUMA; speaker fees from Merck; and licensing fees for intellectual property from Celyad Oncology. S. Takahashi reports grants and/or personal fees from AstraZeneca, Bayer, Chugai, Daiichi Sankyo, Eisai, MSD, Novartis and Taiho. T.B.-S. reports institutional research funding from Abgenomics, Agios, Arcus, Arys, Atreca, Bayer, BMS, Boston Biomedical, Celgene, Clovis, Eisai, Genentech, Incyte, Ipsen, Lilly, Merus, Mirati, Novartis, Pfizer and Seagen; institutional consulting fees from Arcus, Bayer, Eisai, Incyte, Ipsen, Genentech, Merck KGaA, Merck, Merus, Pfizer, Seagen and Servier; personal consulting fees from AbbVie, Aptitude Health, AstraZeneca, Beigene, Blueprint Medicines, Boehringer Ingelheim, Caladrius Biosciences, Celularity, Daiichi Sankyo, Deciphera, Exact Science, Exelixis, Foundation Medicine, GlaxoSmithKline, Illumina, Janssen, Kanaph, MJH Life Sciences, Natera, Sanofi, Sobi, Stemline, TreosBio, Xilio and Zai Labs; IDMC/DSMB roles with 1Globe, AstraZeneca, Exelixis, Fibrogen, Merck/Eisai, PanCan, Suzhou Kintor and The Valley Hospital; consulting or advisory board roles with Artiva, Bard, Immuneering, Imugene, Replimune, Sun Biopharma and Xilis; and patents WO/2018/183488: Human PD1 peptide vaccines and uses thereof, licensed to Imugene, and WO/2019/055687: Methods and compositions for the treatment of cancer cachexia, licensed to Recursion. M.E.B. reports consulting or advisory roles with LifeOmic, Novartis and Strata Oncology; institutional research funding from AbbVie, Apollomics, Arcus Ventures, Elevation Oncology, Endeavor BioMedicines, Genentech, Loxo, Merck, Puma Biotechnology, Seagen and Strata Oncology; travel, accommodations and expenses from LifeOmic; and patents, royalties and other intellectual property: patents for (1) an implantable and localized drug delivery device that can sample the tumor microenvironment and deliver the drug, (2) a method to detect recombination events with CRISPR-mediated editing and (3) expansion microscopy without specialized equipment. K.Y.C. reports institutional research funding from Epizyme+L20 and MedImmune. P.R.D. reports institutional grants from Pfizer; advisory board roles with Astellas Pharma, BMS, Ipsen, Merck and Pfizer; honoraria for lectures from Bayer; travel support from Janssen; and a role as a substitute board member for the Clinical Trials College, Federal Public Service, Kingdom of Belgium; and holds stocks in Alkermes, Mural Oncology PLC and Biocartis Group NV. V.G. reports research funding from Bayer, Boehringer and Roche and institutional funding from Amcure, Astelas, AstraZeneca, Bayer, BeiGene, BMS, FibroGen, Genentech, Lilly, Medimmune, Merck Serono, MSD, Natera, Novartis, Roche, Servier, Sierra Oncology and Takeda. M.G.-M. reports a consulting or advisory role with AstraZeneca and travel, accommodation and expenses from GlaxoSmithKline and MSD Oncology. E.P.H. reports advisory and consulting roles (all payments to the institution) with Accutar Biotechnology, AstraZeneca, Daiichi Sankyo, Eli Lilly, Ellipses Pharma, Entos, Fosun Pharma, Genentech/Roche, Gilead Sciences, Jazz Pharmaceuticals, Jefferies LLC, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Pfizer, Stemline Therapeutics, Tempus Labs, Theratechnologies, Tubulis, Verascity Science and Zentalis Pharmaceuticals and institutional research funding from Abbvie, Accutar Biotechnology, Acerta Pharma, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond, Bliss BioPharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Cascadian Therapeutics, Clovis, Compugen, Context Therapeutics, Cullinan, Curis, CytomX, Daiichi Sankyo, Dana-Farber Cancer Institute, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Eisai, Eli Lilly, Ellipses Pharma, Elucida Oncology, EMD Serono, Fochon Pharmaceuticals, FujiFilm, G1 Therapeutics, Genentech/Roche, Gilead Sciences, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, Inspirna, InventisBio, Jacobio, Karyopharm, K-Group Beta, Kind Pharmaceuticals, Leap Therapeutics, Loxo Oncology, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Mersana, Merus, Millennium, Molecular Templates, Myriad Genetic Laboratories, Novartis, Nucana, Olema, OncoMed, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, Pfizer, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Prelude Therapeutics, ProfoundBio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicine, Rgenix, SeaGen, Sermonix Pharmaceuticals, Shattuck Labs, Silverback Therapeutics, StemCentRx, Stemline Therapeutics, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Zenith Epigenetics and Zymeworks. B.J.M. reports honorarium for consulting or speaking from Acrivon, Adaptimmune, Agenus, Akeso Bio, Amgen, AstraZeneca, Biohaven, BMS, Corcept, Easai, Eli Lilly, Genalux, Genmab/Seagen/Pfizer, GOG Foundation, Gradalis, GSK, Heng Rui, Immunogen/Abbvie, Iovance, Karyopharm, Merck, Mersana, Mural/Alkermes, Myriad, Novartis, Novocure, OncoC4, Panavance, ProfoundBio, Regeneron, Roche/Genentech, Sutro, Verastem, Zentalis and Zymeworks. Y.N. reports consulting or advisory roles with Daiichi Sankyo, Exact Sciences, Gilead Sciences, Guardant Health, Natera, Premo Partners, Roche, Seagen and Takeda; participation in speakers’ bureau for Becton Dickinson, CareNet, Chugai Pharma, Daiichi Sankyo, Eisai, Guardant Health, Guardant Health Japan, Hisamitsu Pharmaceutical, Merck, Miyarisan Pharmaceutical, MSD KK, Taiho Pharmaceutical and Zeria Pharmaceutical; and institutional research funding from Chugai Pharma, Daiichi Sankyo, Genomedia, Guardant Health, Guardant Health AMEA, Roche Diagnostics KK, Seagen and Tempus. D.N. reports a consulting or advisory role with Janssen Oncology; stock and other ownership interests in Intuitive Surgical and Teladoc; and financial interests with Novartis and Takeda. D.M.O. reports consulting or advisory roles with Adaptimmune, Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, DualityBio, Eisai, Elevar Therapeutics, GlaxoSmithKline, GOG Foundation, Immunogen, Imvax, Laekna Therapeutics, Merck, Mersana, Novartis, Novocure, OncoC4, Onconova Therapeutics, Regeneron, Roche, Seagen, Sutro Biopharma, Umoja Biopharma and Verastem and institutional research funding from AbbVie, AbbVie/Stemcentrx, Acerta Pharma, Advaxis, Ajinomoto, Amgen, AstraZeneca, Arcus Biosciences, Array BioPharma, BBI Healthcare, BeiGene, Bristol Myers Squibb, Cerulean Pharma, Clovis Oncology, Deciphera, Eisai, EMD Serono, Ergomed, Exelixis, Genentech/Roche, Genmab, GlaxoSmithKline, OncoQuest, Pfizer, Precision Therapeutics, Immunogen, Incyte, Iovance Biotherapeutics, Janssen Research & Development, Karyopharm Therapeutics, Leap Therapeutics, Ludwig Institute for Cancer Research, Merck, Mersana, Novartis, NovoCure, PharmaMar, Regeneron, Roche, Sanofi, Seagen, Sumitomo Dainippon Pharma Oncology, Sutro Biopharma, Tesaro, TRACON Pharma and Verastem. A.B.O. reports consulting or advisory roles with AstraZeneca, Clovis Oncology, Genentech, GlaxoSmithKline, Novocure and Tesaro. M.R. reports honoraria for lectures and consultancy from Amgen, AstraZeneca, BMS, BeiGene, Boehringer Ingelheim, Daiichi Sankyo, GSK, Lilly, Merck, Mirati, MSD, Novartis, Pfizer, Regeneron and Sanofi; and compensation for membership in DMSB by Daiichi Sankyo and Sanofi. K.S. reports research funds from AstraZeneca, Amgen, Daiichi Sankyo, Gilead, Merck, NanoCarrier, PRA Health Sciences and Takeda; and personal fees from AstraZeneca, Bayer Yakuhin, Eisai, MSD, Nihon Medi-Physics and Pfizer. Y.S. reports institutional grants or contracts from Chugai and Taiho; payment or honoraria for lectures, presentations, speaker’s bureau, paper writing or educational events from Astellas, Bayer Yakuhin, Bristol Myers Squibb KK, Chugai, Daiichi Sankyo, Eli Lilly Japan KK, Guardant Health, Merck, MSD KK, Novartis, Ono, Sysmex, Taiho and Takeda; and roles on data safety monitoring or advisory boards with Guardant Health, Merck and Ono. C.V.M. reports institutional research funding from DigiCore and Gilead; consulting or advisory roles (to the institution) from AstraZeneca, Daiichi Sankyo, Lilly, MSD and Novartis; travel, accommodations and expenses from Amgen, Astellas, Bristol Myers Squibb, DigiCore, Gilead, Merck, MSD Oncology, Pfizer and Roche; and institutional research funding from all companies above. E.Y.Y. reports consulting or advisory roles with AADi, Advanced Accelerator Applications, Bayer, Janssen, Merck and Oncternal Therapeutics; institutional research funding from Agensys, Bayer, Blue Earth Diagnostics, Daiichi Sankyo, Dendreon, Lantheus Medical Imaging, Merck, Seagen, Surface Oncology, Taiho Pharmaceutical and Tyra Biosciences; personal fees from Aadi Bioscience, Advanced Accelerator Applications, Janssen and Oncterna; and grants and personal fees from Bayer and Merck. J.R. reports employment at Pfizer; stock and other ownership interests in Pfizer; and travel and accommodation expenses from Pfizer. S. Tan reports employment at Pfizer; stock and other ownership interests in Pfizer; and travel and accommodation expenses from Pfizer. M.B. reports employment at Pfizer, and stock and other ownership interests in Pfizer. T.E.S. reports consulting or advisory roles with Abbvie, AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Coherus Biosciences, G1 Therapeutics, Gilead Sciences, Pfizer, Spectrum Pharmaceuticals and Takeda and institutional research funding from AstraZeneca, Genentech/Roche, Mirati Therapeutics, Nuvalent, and Seagen. P.R.P. reports honoraria from Dava Oncology, OncLive and Frontiers; consulting or Advisory Role with Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, Heron, Puma Biotechnology, Sirtex Medical, Caris Life Sciences, Juniper Pharmaceuticals, Bolt Biotherapeutics and AbbVie; speakers’ bureau with Genentech/Roche; institutional research funding from Genentech/Roche, Fabre-Kramer, Advanced Cancer Therapeutics, Caris Centers of Excellence, Pfizer, Pieris Pharmaceuticals, Cascadian Therapeutics, Bolt Biotherapeutics, Byondis, Seagen, Orum and Carisma; and intellectual property US patent numbers 8,486,413, 8,501,417, 9,023,362 and 9,745,377. Non-financial interests: G.C. reports uncompensated relationships with Consiglio Superiore di Sanità (officer), Italian National Health Council (advisor for the Ministry of Health), ESMO (officer), ESMO Open (editor in chief), Europa Donna (advisory role), member of the Scientific Council Patient Advocacy Association, EUSOMA (leadership role) and Fondazione Beretta (advisory role) Cancer Research Foundation. A.R. reports research funding from Bristol Myers Squibb; payment or honoraria from Bristol Myers Squibb and Novartis; and support for attending meetings and/or travel from Bristol Myers Squibb, Merck Sharp & Dohme and Novartis. D.N. reports uncompensated relationships with Takeda and Novartis. C.V.M. reports a non-financial competing interest as a board member of BSMO. P.R.P. reports uncompensated relationships with Pfizer, Seagen and Jazz.
Figures

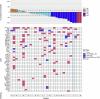
References
-
- Riese, D. J. 2nd & Stern, D. F. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays20, 41–48 (1998). - PubMed
-
- Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol.2, 127–137 (2001). - PubMed
-
- Schlessinger, J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell110, 669–672 (2002). - PubMed
-
- Holbro, T. & Hynes, N. E. ErbB receptors: directing key signaling networks throughout life. Annu. Rev. Pharmacol. Toxicol.44, 195–217 (2004). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

