Inter-Rater Disagreements in Applying the Montreal Classification for Crohn's Disease: The Five-Nations Survey Study
- PMID: 39825768
- PMCID: PMC12188366
- DOI: 10.1002/ueg2.12757
Inter-Rater Disagreements in Applying the Montreal Classification for Crohn's Disease: The Five-Nations Survey Study
Abstract
Background: The Montreal classification has been widely used in Crohn's disease since 2005 to categorize patients by the age of onset (A), disease location (L), behavior (B), and upper gastrointestinal tract and perianal involvement. With evolving management paradigms in Crohn's disease, we aimed to assess the performance of gastroenterologists in applying the Montreal classification.
Methods: An online survey was conducted among participants at an international educational conference on inflammatory bowel diseases. Participants classified 20 theoretical Crohn's disease cases using the Montreal classification. Agreement rates with the inflammatory bowel diseases board (three expert gastroenterologists whose consensus rating was considered the gold standard) were calculated for gastroenterologist specialists and fellows/specialists with ≤ 2 years of clinical experience. A majority vote < 75% among participants was considered a notable disagreement. The same cases were classified using three large language models (LLMs), ChatGPT-4, Claude-3, and Gemini-1.5, and assessed for agreement with the board and gastroenterologists. Fleiss Kappa was used to assess within-group agreement.
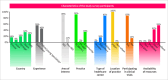
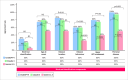
Results: Thirty-eight participants from five countries completed the survey. In defining the Montreal classification as a whole, specialists (21/38 [55%]) had a higher agreement rate with the board compared to fellows/young specialists (17/38 [45%]) (58% vs. 49%, p = 0.012) and to LLMs (58% vs. 18%, p < 0.001). Disease behavior classification was the most challenging, with 76% agreement among specialists and fellows/young specialists and 48% among LLMs compared to the inflammatory bowel diseases board. Regarding disease behavior, within-group agreement was moderate (specialists: k = 0.522, fellows/young specialists: k = 0.532, LLMs: k = 0.577; p < 0.001 for all). Notable points of disagreement included: defining disease behavior concerning obstructive symptoms, assessing disease extent via video capsule endoscopy, and evaluating treatment-related reversibility of the disease phenotype.
Conclusions: There is significant inter-rater disagreement in applying the Montreal classification, particularly for disease behavior in Crohn's disease. Improved education or revisions to phenotype criteria may be needed to enhance consensus on the Montreal classification.
Keywords: Crohn's disease; complicated disease phenotype; inflammatory bowel diseases; large language models; montreal classification.
© 2025 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
NKD received honoraria from Abbvie, Takeda and Cadigroup. CGM has received educational funding from Abbvie, Janssen, Kern Pharma, MSD, Tillots Pharma and has served as speaker for Galapagos. AA (Alessandro Armuzzi) received Consulting/advisory board fees from AbbVie, Alfa‐Sigma, Amgen, Astra Zeneca, Biogen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celltrion, Eli‐Lilly, Ferring, Galapagos, Gilead, Giuliani, Janssen, Lionhealth, Merck, Nestlé, Pfizer, Protagonist Therapeutics, Roche, Sanofi, Samsung Bioepis, Sandoz, Takeda, Tillots Pharma; Speaker’s fees from AbbVie, AG Pharma, Amgen, Biogen, Bristol‐Myers Squibb, Celltrion, Eli‐Lilly, Ferring, Galapagos, Gilead, Janssen, Lionealth, Merck, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, Teva Pharmaceuticals. SBH has received advisory board and/or consulting fees from Abbvie, Takeda, Janssen, Celltrion, Pfizer, GSK, Ferring, Novartis, Roche, Gilead, NeoPharm, Predicta Med, Galmed, Medial Earlysign, BMS and Eli Lilly, holds stocks/options in Predicta Med, Evinature & Galmed, and received research support from Abbvie, Takeda, Janssen, Celltrion, Pfizer, & Galmed. UK received speaker and consultancy fees from Abbvie, BMS, Elly Lilly, Celtrion, Medtronic, Janssen and, Pfizer, Roche and Takeda, research support from Abbvie, Elli Lilly, Medtronic Takeda and Janssen. DL has received counseling, boards, transports or fees from Abbvie, Amgen, Biogaran, Biogen, Celltrion, Ferring, Galapagos, Janssen, Lilly, Medac, MSD, Pfizer, Prometheus, Sandoz, Takeda, Theradiag. HY received Advisory Committee or Review Panel fees from Takeda, Abbvie, Pfizer, Janssen, BMS, and Eli Lilli; Speaking and Teaching fees from Abbvie, Pfizer, Takeda, Novartis, and BMS; Grant/Research Support from Pfizer. NR has received lecture and consultancy fees from AbbVie, Janssen, and Takeda. FM received Lectures fees from AbbVie, Amgen, Biogen, Bristol Myers Squibb/Celgene, Celltrion, Dr Falk Foundation, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Janssen, Lilly, MSD, Pfizer, Sandoz/Hexal, Takeda, Tillotts, and Vifor Pharma, Laboratorios Victoria. YZ have received support for conference attendance, speaker fees, research support and consulting fees from AbbVie, Adacyte Therapeutics, Almirall, Amgen, Dr. Falk Pharma, Faes Farma, Ferring Pharmaceuticals, Janssen, MSD, Otsuka, Pfizer, Shire, Takeda, Galapagos, Boehringer Ingelheim, Sanofi, Fresenius Kabi, Alfa‐Sigma and Tillotts Pharma AG. MBA has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Takeda, Galapagos‐Alpha Sigma, Pfizer, Sandoz, Fresenius, Lilly, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Adacyte and TillotsPharma. AA (Aurelien Amiot) received consulting fees from Abbvie, Pfizer, Takeda, Tillotts Pharma, Janssen and Sandoz as well as lecture fees and travel accommodations from Abbvie, Janssen, Pfizer, Takeda, Biogen, Fresenius Kabi, Amgen and Celltrion. LM has served as a speaker, consultant or advisory member for or has received unrestricted grants from MSD, Abbvie, Takeda, Janssen, Pfizer, Biogen, Galapagos, Kern Pharma, Lilly, Otsuka Pharmaceuticals, Tillotts, Dr. Falk Pharma, Ferring, Medtronic and General Electric. GD reports speaker fees and/or consultancy fees from Alfasigma, Celltrion Healthcare, Ferring, Johnson&Johnson, Novartis, Pfizer, and Takeda. HTS received speaker fees and travel accommodations from AbbVie, Biogen, Dr Falk Foundation, Ferring Pharmaceuticals, Janssen, Lilly, MSD, Pfizer, Takeda, Tillotts, and Laboratorios Victoria. The remaining authors declare no conflicts of interest.
Figures

References
-
- Ramadas A. V., Gunesh S., Thomas G. A. O., Williams G. T., and Hawthorne A. B., “Natural History of Crohn’s Disease in a Population‐Based Cohort From Cardiff (1986–2003): A Study of Changes in Medical Treatment and Surgical Resection Rates,” Gut 59, no. 9 (2010): 1200–1206, 10.1136/gut.2009.202101. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

