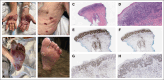
Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma with novel FGFR1 fusion treated with pemigatinib
- PMID: 39825823
- PMCID: PMC12008514
- DOI: 10.1182/bloodadvances.2024014928
Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma with novel FGFR1 fusion treated with pemigatinib
Conflict of interest statement
Figures
Similar articles
-
[Facial ulcerated nodules revealing primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma].Ann Dermatol Venereol. 2020 Nov;147(11):764-768. doi: 10.1016/j.annder.2020.04.014. Epub 2020 Jun 8. Ann Dermatol Venereol. 2020. PMID: 32527516 French.
-
Vulvar Primary Cutaneous CD8+ Aggressive Epidermotropic Cytotoxic T-Cell Lymphoma.Int J Gynecol Pathol. 2021 May 1;40(3):229-233. doi: 10.1097/PGP.0000000000000648. Int J Gynecol Pathol. 2021. PMID: 33741766
-
Primary cutaneous aggressive epidermotropic CD8(+) cytotoxic T-cell lymphoma with atypical presentation.J Dermatol. 2006 Sep;33(9):632-4. doi: 10.1111/j.1346-8138.2006.00147.x. J Dermatol. 2006. PMID: 16958809
-
Aggressive epidermotropic cutaneous CD8+ lymphoma: a cutaneous lymphoma with distinct clinical and pathological features. Report of an EORTC Cutaneous Lymphoma Task Force Workshop.Histopathology. 2015 Oct;67(4):425-41. doi: 10.1111/his.12371. Epub 2015 Feb 24. Histopathology. 2015. PMID: 24438036
-
Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma.J Am Acad Dermatol. 2010 Feb;62(2):300-7. doi: 10.1016/j.jaad.2009.02.035. Epub 2009 Nov 26. J Am Acad Dermatol. 2010. PMID: 19944484 Review.
Cited by
-
TRAF3IP3::FGFR1: a novel FGFR1 fusion identified in an aggressive case of acute myeloid leukemia.Ann Hematol. 2025 Jul 1. doi: 10.1007/s00277-025-06494-9. Online ahead of print. Ann Hematol. 2025. PMID: 40590913 No abstract available.
References
-
- Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479–1484. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

