Final analysis of the phase 1b Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT)
- PMID: 39825857
- PMCID: PMC12008623
- DOI: 10.1182/bloodadvances.2024014900
Final analysis of the phase 1b Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT)
Abstract
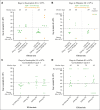
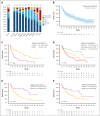
Venetoclax plus azacitidine represents a key advance for older, unfit patients with acute myeloid leukemia (AML). The Chemotherapy and Venetoclax in Elderly AML Trial (CAVEAT) was first to combine venetoclax with intensive chemotherapy in newly diagnosed patients aged ≥65 years. In this final analysis, 85 patients (median age, 71 years) were followed up for a median of 41.8 months. The CAVEAT induction combined cytarabine and idarubicin with 5 dose levels of venetoclax (50-600 mg) for up to 14 days. Two additional cohorts explored adjusted-dose venetoclax (50 mg and 100 mg) with posaconazole. CAVEAT induction was well tolerated, with low mortality (4%) and limited high-grade gastrointestinal toxicity (4%). Delayed hematologic recovery after consolidation was ameliorated by omitting idarubicin from postremission therapy. The overall response rate (ORR; complete response [CR] + CR with partial hematologic recovery + CR with incomplete count recovery) was 75%, with a median overall survival (OS) of 19.3 months (95% confidence interval [CI], 11.1-31.3). Among de novo AML, ORR was 88% and median OS was 33.1 months (95% CI, 19.3-54.3). Almost one-third have not relapsed, many benefiting from prolonged treatment-free remission (median, 17.9 months). CAVEAT induction was well tolerated and associated with high ORR that was durable, particularly for de novo AML. CAVEAT represents an effective time-limited treatment option for fit, older patients with AML. This trial was registered at https://www.anzctr.org.au as #ACTRN12616000445471.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.C.C. has participated in advisory board meetings for AbbVie, Pfizer, and Sumitomo Pharma Oncology, and received honoraria from Otsuka, Bristol Myers Squibb (BMS), AstraZeneca, AbbVie, and Pfizer. S.L. has received honoraria for speaker's bureau from AbbVie. I.S.T. has received honoraria from Jazz Pharmaceuticals, Novartis, and Pfizer. C.Y.F. has consulted for, served on advisory boards of, or received honoraria for speakers bureau from Astellas, AbbVie, BeiGene, BMS, Jazz Pharmaceuticals, Limbic, Novartis, Novotech, Otsuka, and Pfizer, and received research funding from Jazz Pharmaceuticals and Astellas. S.F. has consulted for, served on advisory boards of, and received honoraria for speakers' bureaus from Astellas, AbbVie, Amgen, BMS, Gilead/Kite, Jazz Pharmaceuticals, Limbic, Novartis, and Pfizer, and has received research funding from Amgen. J.R. holds stock in Novartis AG, Sandoz AG, and Alcon AG, and his employer, Alfred Health, receives funds from AbbVie for his involvement in 1 research project. A.H.W. has served on advisory boards for Novartis, AstraZeneca, Astellas, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, BMS, and BeiGene; has consulted for AbbVie, Servier, Novartis, Shoreline, and Aculeus; receives research funding to the institution from Novartis, AbbVie, Servier, BMS, Janssen, Syndax, Astex, AstraZeneca, and Amgen; and serves on speakers' bureaus for AbbVie, Novartis, BMS, Servier, and Astellas. A.H.W., A.W.R., and N.S.A. are employees of the Walter and Eliza Hall Institute (WEHI). WEHI receives milestone and royalty payments related to the development of venetoclax. Current and past employees of WEHI may be eligible for financial benefits related to these payments. A.H.W., A.W.R., and N.S.A. receive such a financial benefit. A.W.R. is an inventor on a patent related to venetoclax. The remaining authors declare no competing financial interests.
Figures


References
-
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. - PubMed
-
- Chua CC, Roberts AW, Reynolds J, et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): a phase Ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J Clin Oncol. 2020;38(30):3506–3517. - PubMed
-
- Gardin C, Turlure P, Fagot T, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy:results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109(12):5129–5135. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

