PRMT5 inhibition reduces hyperinflammation in a murine model of secondary hemophagocytic lymphohistiocytosis
- PMID: 39825858
- PMCID: PMC12141904
- DOI: 10.1182/bloodadvances.2024013651
PRMT5 inhibition reduces hyperinflammation in a murine model of secondary hemophagocytic lymphohistiocytosis
Abstract
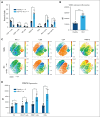
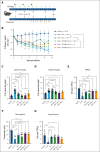
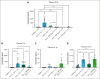
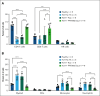
Hemophagocytic lymphohistiocytosis (HLH) is a rare but aggressive and potentially lethal hyperinflammatory syndrome characterized by pathologic immune activation and excessive production of proinflammatory cytokines leading to tissue damage and multisystem organ failure. There is an urgent need for the discovery of novel targets and development of therapeutic strategies to treat this rare but deadly syndrome. Protein arginine methyltransferase 5 (PRMT5) mediates T-cell-based inflammatory responses, making it a potential actionable target for the treatment of HLH. Using CPG-1826 and anti-interleukin-10R (IL-10R) antibody, we induced murine secondary HLH in vivo with a marked expansion of splenic myeloid cell subsets and concurrent reduction of T- and natural killer (NK)-cell populations. PRMT5 expression was significantly upregulated in splenic T and NK lymphocytes, monocytes, and dendritic cells in mice with HLH (P < .05). Treatment with PRT382, a potent and selective PRMT5 inhibitor, significantly reduced physical signs of secondary HLH, including splenomegaly, hepatomegaly, and anemia (P < .0001 in each case), when compared with untreated mice. Inflammatory cytokines known to drive hyperinflammation in HLH, including interferon-γ and IL-6 were reduced to healthy levels with PRT382 treatment (P > .999 for both). PRT382 treatment also reduced the expansion of myeloid cell populations (P < .0001) in mice with HLH, compared with untreated mice, while restoring T- and NK-cell numbers (P < .001 for both). These results identify PRMT5 as a promising target for the management of secondary HLH and justify further exploration in this and other models of hyperinflammation.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P. Scherle, N.B., and K.V. report employment with Prelude Therapeutics. R.A.B. received research support for PRT drug product from Prelude Therapeutics. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Breaking the cycle: PRMTs and secondary HLH relief.Blood Adv. 2025 May 27;9(10):2436-2437. doi: 10.1182/bloodadvances.2025015863. Blood Adv. 2025. PMID: 40372747 Free PMC article. No abstract available.
References
-
- Bhatt NS, Oshrine B, An Talano J. Hemophagocytic lymphohistiocytosis in adults. Leuk Lymphoma. 2019;60(1):19–28. - PubMed
-
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. - PubMed
-
- Gupta S, Weitzman S. Primary and secondary hemophagocytic lymphohistiocytosis: clinical features, pathogenesis and therapy. Expet Rev Clin Immunol. 2010;6(1):137–154. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

