Definitions and use of tumor bulk in phase 3 lymphoma trials: a comprehensive literature review
- PMID: 39825862
- PMCID: PMC12124615
- DOI: 10.1182/bloodadvances.2024015072
Definitions and use of tumor bulk in phase 3 lymphoma trials: a comprehensive literature review
Abstract
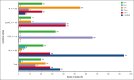
Tumor "bulk" has historically been considered an important prognostic marker and a clinical tool to guide treatment in patients with lymphoma. However, its use and definitions in trial designs vary significantly, and it is unclear how this has influenced the relevance of bulk in contemporary practice. This comprehensive literature review evaluated the definitions, applications, and prognostic impact of bulk in phase 3 randomized trials in 4 major lymphoma subtypes. Overall, 87 studies were identified across follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), peripheral T-cell lymphoma (PTCL), and Hodgkin lymphoma (HL) with a wide range of bulk thresholds used (5 cm, 6 cm, 7 cm, 7.5 cm, 10 cm, and >1/3 mediastinal mass ratio [MMR]). The most common threshold was as follows: FL, 7 cm (58%); DLBCL, 7.5 cm and 10 cm (44% each); PTCL, 7.5 cm (66%); and HL, one-third MMR (91%). Bulk threshold was used by trials to determine eligibility (66%), stratification (24%), as a prognostic risk factor (37%), and as a decision tool for risk-adapted treatment, for example, radiotherapy (29%); however, bulk definitions used for these varied both between, and within, lymphoma subtypes and even within single trials in 25%. Furthermore, 32 studies incorporated bulk in prognostic analyses with only 5 showing significance for differential survival outcomes. Our analysis demonstrates high inconsistency in thresholds defining tumor bulk and use of bulk in phase 3 lymphoma trials across eligibility, stratification, therapeutic risk adaptation, and prognostication. This highlights an urgent need for international consensus on definitions of bulk within trials to improve its prognostic and predictive values and refine its application in clinical practice.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.A.H. received research funding (paid to institution) from Roche, Bristol Myers Squibb (BMS), Merck KGaA, AstraZeneca, TG Therapeutics, and Merck; received travel expenses from AstraZeneca; and is involved in consultant or advisory roles (∗paid to institution) for Roche∗, Merck Sharpe & Dohme∗ (MSD), AstraZeneca∗, Gilead, Antengene∗, Novartis∗, Regeneron, Janssen∗, Specialised Therapeutics∗, and Sobi∗. E.M.W. received research funding (paid to institution) from Abbvie, Amgen, Antengene, AstraZeneca, Beigene, Bristol-Myers Squibb, Celgene, CSL Behring, Gilead, GSK, Janssen-Cilag, Novartis, Pfizer, Roche, Sanofi, Sobi and Takeda. Z.K.M. received research funding (paid to institution) from Abbvie, Amgen, Antengene, AstraZeneca, Beigene, Bristol-Myers Squibb, Celgene, CSL Behring, Gilead, GSK, Janssen-Cilag, Novartis, Pfizer, Roche, Sanofi, Sobi and Takeda. P.R.D.C. received honoraria from Janssen, AbbVie, and Gilead; and travel expenses from Gilead. G.P.G. received research funding (paid to institution) from Janssen, AbbVie, BeiGene, and Merck; received honoraria from Novartis, Janssen, Roche, BMS, MSD, Clinigen, and Prelude Therapeutics. C.T. received honoraria from Janssen, AbbVie, BeiGene, Loxo, and AstraZeneca; received research funding from Janssen, AbbVie, and BeiGene. The remaining authors declare no competing financial interests.
Figures
References
-
- Schomberg PJ, Evans RG, O'Connell MJ, et al. Prognostic significance of mediastinal mass in adult Hodgkin's disease. Cancer. 1984;53(2):324–328. - PubMed
-
- Gospodarowicz MK, Bush RS, Brown TC, Chua T. Prognostic factors in nodular lymphomas: a multivariate analysis based on the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys. 1984;10(4):489–497. - PubMed
-
- Wilder RB, Rodriguez MA, Ha CS, et al. Bulky disease is an adverse prognostic factor in patients treated with chemotherapy comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy for aggressive lymphoma. Cancer. 2001;91(12):2440–2446. - PubMed
-
- Meignan M, Cottereau AS, Versari A, et al. Baseline metabolic tumor volume predicts outcome in high–tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;34(30):3618–3626. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical