Digging deeper into necrotizing enterocolitis: bridging clinical, microbial, and molecular perspectives
- PMID: 39826099
- PMCID: PMC12128673
- DOI: 10.1080/19490976.2025.2451071
Digging deeper into necrotizing enterocolitis: bridging clinical, microbial, and molecular perspectives
Abstract
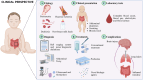
Necrotizing Enterocolitis (NEC) is a severe, life-threatening inflammatory condition of the gastrointestinal tract, especially affecting preterm infants. This review consolidates evidence from various biomedical disciplines to elucidate the complex pathogenesis of NEC, integrating insights from clinical, microbial, and molecular perspectives. It emphasizes the modulation of NEC-associated inflammatory pathways by probiotics and novel biologics, highlighting their therapeutic potential. We further critically examine dysbiotic alterations within the gut microbiota, with a particular focus on imbalances in bacterial and viral communities, which may contribute to the onset of NEC. The intricate interactions among toll-like receptor 4 (TLR4), microvascular integrity, immune activation, and the inflammatory milieu are meticulously summarized, offering a sophisticated understanding of NEC pathophysiology. This academic review aims to enhance the etiological comprehension of NEC, promote the development of targeted therapeutic interventions, and impart the significant impact of perinatal factors on the formulation of preventive and curative strategies for the disease.
Keywords: Necrotizing enterocolitis (NEC); dysbiosis; mechanism; microbes; preterm infants; toll-like receptor 4 (TLR4).
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
