HER2DX in HER2-positive inflammatory breast cancer: correlative insights and comparative analysis with noninflammatory breast cancers
- PMID: 39826476
- PMCID: PMC11786065
- DOI: 10.1016/j.esmoop.2024.104100
HER2DX in HER2-positive inflammatory breast cancer: correlative insights and comparative analysis with noninflammatory breast cancers
Abstract
Background: The HER2DX assay predicts long-term prognosis and pathologic complete response (pCR) in patients with early-stage human epidermal growth factor receptor 2 (HER2)-positive breast cancer receiving neoadjuvant systemic therapy but has not been evaluated in inflammatory breast cancer (IBC).
Patients and methods: HER2DX was analyzed in baseline biopsy tissues from 23 patients with stage III HER2-positive IBC on a phase II trial (NCT01796197) treated with neoadjuvant trastuzumab, pertuzumab, and paclitaxel (THP). To assess the assay's predictive accuracy for pCR in IBC, clinical-pathological features and outcomes from this IBC cohort were compared with 156 patients with stage III HER2-positive non-IBC from four different cohorts. Comparative analyses included HER2DX scores, gene signatures, and expression of individual genes between patients with IBC and non-IBC.
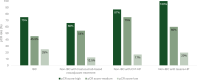
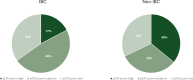
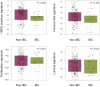
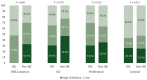
Results: Notable differences in clinicopathological characteristics included higher pertuzumab and chemotherapy usage and lower axillary burden in patients with IBC compared with non-IBC. In the combined cohort (n = 179), HER2DX pCR score and pertuzumab use were significant predictors of pCR, but not IBC status. The pCR rates in patients treated with trastuzumab-based chemotherapy (including IBC and non-IBC) were 68.9%, 58.5%, and 16.3% in the HER2DX pCR-high, -medium, and -low groups, respectively. Comparative gene expression analysis indicated minor differences between IBC and non-IBC affecting individual HER2, immune, and proliferation genes.
Conclusions: The HER2DX pCR score could predict pCR in stage III HER2-positive IBC following treatment with de-escalated neoadjuvant systemic therapy and in stage III HER2-positive non-IBC. Elevated pCR rates in HER2-positive IBC with high HER2DX pCR scores suggest there may be a role for treatment de-escalation in these patients and confirmatory studies are justified.
Keywords: HER2-positive; HER2DX; inflammatory breast cancer; pathologic complete response.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Chang S., Parker S.L., Pham T., Buzdar A.U., Hursting S.D. Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program of the National Cancer Institute, 1975-1992. Cancer. 1998;82(12):2366–2372. - PubMed
-
- Abraham H.G., Xia Y., Mukherjee B., Merajver S.D. Incidence and survival of inflammatory breast cancer between 1973 and 2015 in the SEER database. Breast Cancer Res Treat. 2021;185(1):229–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

