Profiling Tel1 signaling reveals a non-canonical motif targeting DNA repair and telomere control machineries
- PMID: 39826692
- PMCID: PMC11875207
- DOI: 10.1016/j.jbc.2025.108194
Profiling Tel1 signaling reveals a non-canonical motif targeting DNA repair and telomere control machineries
Abstract
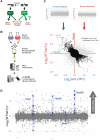
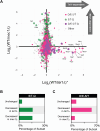
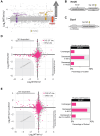
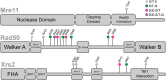
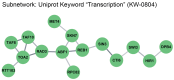
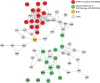
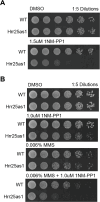
The stability of the genome relies on phosphatidyl inositol 3-kinase-related kinases (PIKKs) that sense DNA damage and trigger elaborate downstream signaling responses. In Saccharomyces cerevisiae, the Tel1 kinase (ortholog of human ATM) is activated at DNA double-strand breaks (DSBs) and short telomeres. Despite the well-established roles of Tel1 in the control of telomere maintenance, suppression of chromosomal rearrangements, activation of cell cycle checkpoints, and repair of DSBs, the substrates through which Tel1 controls these processes remain incompletely understood. Here we performed an in-depth phosphoproteomic screen for Tel1-dependent phosphorylation events. To achieve maximal coverage of the phosphoproteome, we developed a scaled-up approach that accommodates large amounts of protein extracts and chromatographic fractions. Compared to previous reports, we expanded the number of detected Tel1-dependent phosphorylation events by over 10-fold. Surprisingly, in addition to the identification of phosphorylation sites featuring the canonical motif for Tel1 phosphorylation (S/T-Q), the results revealed a novel motif (D/E-S/T) highly prevalent and enriched in the set of Tel1-dependent events. This motif is unique to Tel1 signaling and not shared with the Mec1 kinase, providing clues to how Tel1 plays specialized roles in DNA repair and telomere length control. Overall, these findings define a Tel1-signaling network targeting numerous proteins involved in DNA repair, chromatin regulation, and telomere maintenance that represents a framework for dissecting the molecular mechanisms of Tel1 action.
Keywords: DNA damage signaling; DNA repair; Tel1; kinase signaling; mass spectrometry; phosphoproteomics; phosphorylation; telomere maintenance.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Update of
-
Profiling Tel1 Signaling Reveals a Non-Canonical Motif Targeting DNA Repair and Telomere Control Machineries.bioRxiv [Preprint]. 2024 Jul 5:2024.07.03.601872. doi: 10.1101/2024.07.03.601872. bioRxiv. 2024. Update in: J Biol Chem. 2025 Mar;301(3):108194. doi: 10.1016/j.jbc.2025.108194. PMID: 39005478 Free PMC article. Updated. Preprint.
References
-
- Jia-Lin Ma N., Stern D.F. Regulation of the Rad53 protein kinase in signal amplification by oligomer assembly and disassembly. Cell Cycle. 2008;7:808–817. - PubMed
-
- Kumagai A., Dunphy W.G. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell. 2000;6:839–849. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

