Advances in cardiac organoid research: implications for cardiovascular disease treatment
- PMID: 39827092
- PMCID: PMC11743075
- DOI: 10.1186/s12933-025-02598-8
Advances in cardiac organoid research: implications for cardiovascular disease treatment
Abstract
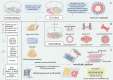
Globally, cardiovascular diseases remain among the leading causes of mortality, highlighting the urgent need for innovative research models. Consequently, the development of accurate models that simulate cardiac function holds significant scientific and clinical value for both disease research and therapeutic interventions. Cardiac organoids, which are three-dimensional structures derived from the induced differentiation of stem cells, are particularly promising. These organoids not only replicate the autonomous beating and essential electrophysiological properties of the heart but are also widely employed in studies related to cardiac diseases, drug efficacy testing, and regenerative medicine. This review comprehensively surveys the various fabrication techniques used to create cardiac organoids and their diverse applications in modeling a range of cardiac diseases. We emphasize the role of advanced technologies in enhancing the maturation and functionality of cardiac cells, ensuring that these models closely resemble native cardiac tissue. Furthermore, we discuss monitoring techniques and evaluation parameters critical for assessing the performance of cardiac organoids, considering the complex interactions within multi-organ systems. This approach is vital for enhancing precision and efficiency in drug development, allowing for more effective therapeutic strategies. Ultimately, this review aims to provide a thorough and innovative perspective on both fundamental research and clinical treatment of cardiovascular diseases, offering insights that could pave the way for future advancements in understanding and addressing these prevalent health challenges.
Keywords: Cardiac diseases; Cardiac organoids; Monitoring techniques; Multi-organ systems.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60:727–800. - DOI - PubMed
-
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, de Moraes G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. - DOI - PMC - PubMed
-
- Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, Gale CP, Maggioni AP, Petersen SE, Huculeci R, Kazakiewicz D, de Benito Rubio V, Ignatiuk B, Raisi-Estabragh Z, Pawlak A, Karagiannidis E, Treskes R, Gaita D, Beltrame JF, McConnachie A, Bardinet I, Graham I, Flather M, Elliott P, Mossialos EA, Weidinger F, Achenbach S. European Society of C, on behalf of the Atlas Writing G. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022;43:716–99.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

