ICAM1 blockade improves ischemic muscle reperfusion in diabetic mice
- PMID: 39827135
- PMCID: PMC11742775
- DOI: 10.1186/s12933-025-02573-3
ICAM1 blockade improves ischemic muscle reperfusion in diabetic mice
Abstract
Background: Chronic Limb-Threatening Ischemia (CLTI) represents the most advanced stage of Peripheral Artery Disease (PAD) and is associated with dire prognosis, characterized by a substantial risk of limb amputation and diminished life expectancy. Despite significant advancements in therapeutic interventions, the underlying mechanisms precipitating the progression of PAD to CLTI remain elusive.
Methods: Considering diabetes is one of the main risk factors contributing to PAD exacerbation into CLTI, we compared hind limb ischemia recovery in HFD STZ vs. non-HFD STZ mice to identify new mechanisms responsible for the exacerbation of PAD.
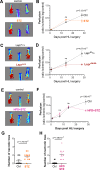
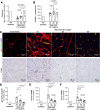
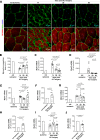
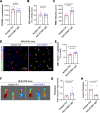
Results: We used three different mouse models of diabetes and found that blood flow recovery in HFD STZ mice is altered only from day 14 post-surgery. Consistent with this kinetics, we found that angiogenesis and myogenesis which typically occur between day five and day 14 post-surgery are not impaired in mice in which diabetes was induced by a high fat diet and streptozotocin injections (HFD STZ mice). On the contrary, we found that capillary functionality e.i. acquisition of functional intercellular junctions and immune quiescence is impaired in HFD + STZ mice. Notably, 28 days after hind limb ischemia surgery, HFD + STZ mice display significantly increased capillary permeability to IgG and significantly increased levels of ICAM1. This was associated with an increased macrophage infiltration and an impaired myocyte differentiation. Importantly, we used ICAM1-blocking antibodies to demonstrate that increased ICAM1 expression in HFD + STZ mice decreases white blood cell circulation velocity within the microcirculation, which impairs its perfusion. Notably anti-ICAM1 therapy did diminish macrophage infiltration and oxidative stress but not myopathy suggesting that myopathy characterized by small myocytes expressing higher level of MYH2 could be responsible for microangiopathy.
Conclusion: ICAM1 expression by the microvasculature impairs ischemic muscle reperfusion in HFD + STZ mice. Importantly, the increase in blood flow between day 14 and day 90 post-HLI surgery is not associated with an increased capillary density but with an improved functionality of capillaries.
Keywords: Endothelial activation; Limb ischemia; Microcirculation; Perfusion.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, McDermott MM, Misra S, Ujueta F, on behalf of the American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation [Internet]; 144, (2021). Accessed 24 Jul 2022. 10.1161/CIR.0000000000001005 - PMC - PubMed
-
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Global Health. 2019;7:e1020–30. - PubMed
-
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V, Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton A, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis JR, Catapano AL, Chugh S, Cooper LT, Coresh J, Criqui MH, DeCleene NK, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Sola J, Fowkes FGR, Gakidou E, Grundy SM, He FJ et al. Global Burden of cardiovascular diseases and risk factors. J Am Coll Cardiol. 2020;76:2982–3021. - PMC - PubMed
-
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

