Maraviroc/cisplatin combination inhibits gastric cancer tumoroid growth and improves mice survival
- PMID: 39827154
- PMCID: PMC11748569
- DOI: 10.1186/s40659-024-00581-3
Maraviroc/cisplatin combination inhibits gastric cancer tumoroid growth and improves mice survival
Abstract
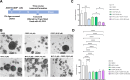
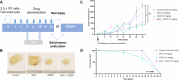
Background: Gastric cancer (GC) is a significant cancer-related cause of death worldwide. GC's most used chemotherapeutic regimen is based on platinum drugs such as cisplatin (CDDP). However, CDDP chemoresistance reduces the survival rate of advanced GC. The immune C-C chemokine receptor type 5 (CCR5) have been proposed as a pivotal factor in cancer progression since its blockade has been linked with antineoplastic effects on tumor cell proliferation; nevertheless, its role in the chemoresistance of GC has not been elucidated. This study aimed to determine the effects induced by the CCR5 using Maraviroc (MVC), a highly selective CCR5 antagonist, on CDDP-resistant AGS cells (AGS R-CDDP), tumoroids (3D tumor spheroids), and animal models.
Results: The combined CDDP and MVC treatment reduced cell viability and inhibited tumoroid formation in AGS R-CDDP cells. The effects of the MVC/CDDP combination on apoptosis and cell cycle progression were correlated with the increase in CDDP (dose-dependent). The mRNA levels of C-C Motif Chemokine Ligand 5 (CCL5), the main ligand for CCR5, decreased significantly in cells treated with the MVC/CDDP combination. MVC in the MVC/CDDP combination improved the survival rate and biochemical parameters of CDDP-treated mice by reducing the side effects of CDDP alone.
Conclusions: This finding suggests that MVC/CDDP combination could be a potential complementary therapy for GC.
Keywords: C-C chemokine receptor type 5 (CCR5); C-C motif chemokine ligand 5 (CCL5); Chemoresistance; Cisplatin (CDDP); Gastric cancer (GC); Maraviroc (MVC).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was approved by the Ethical Committee of Universidad de La Frontera (Approval certificate Nº020/2021) and Ethical Committee of Universidad del Desarrollo (Approval certificate Nº07/2021_CICUAL-UDD). Patents: These results of this study are protected by a patent application filed with the Instituto Nacional de Propiedad Industrial (INAPI) under Application number PCT/CL2024/050006. Consent for publication: Not applicable. Competing interests: There is a patent pending issued. The results of this study are protected by a patent application filed with the Instituto Nacional de Propiedad Industrial (INAPI) under Application number PCT/CL2024/050006.
Figures




Similar articles
-
Synergistic Inhibition of R5 HIV-1 by the Fusion Protein (FLSC) IgG1 Fc and Maraviroc in Primary Cells: Implications for Prevention and Treatment.Curr HIV Res. 2016;14(1):24-36. doi: 10.2174/1570162x13666150909145150. Curr HIV Res. 2016. PMID: 26354735
-
Functional and transcriptomic characterization of cisplatin-resistant AGS and MKN-28 gastric cancer cell lines.PLoS One. 2020 Jan 28;15(1):e0228331. doi: 10.1371/journal.pone.0228331. eCollection 2020. PLoS One. 2020. PMID: 31990955 Free PMC article.
-
Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model.PLoS One. 2013;8(1):e53992. doi: 10.1371/journal.pone.0053992. Epub 2013 Jan 9. PLoS One. 2013. PMID: 23326556 Free PMC article.
-
Maraviroc: a review of its use in HIV infection and beyond.Drug Des Devel Ther. 2015 Oct 1;9:5447-68. doi: 10.2147/DDDT.S90580. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26491256 Free PMC article. Review.
-
Chemokine receptor CCR5 antagonist maraviroc: medicinal chemistry and clinical applications.Curr Top Med Chem. 2014;14(13):1504-14. doi: 10.2174/1568026614666140827143745. Curr Top Med Chem. 2014. PMID: 25159165 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Marin JJG, Al-Abdulla R, Lozano E, Briz O, Bujanda L, Banales M. Mechanisms of resistance to Chemotherapy in Gastric Cancer. Anticancer Agents Med Chem. 2016;16(3):318–34. - PubMed
-
- Zhang D, Fan D. New insights into the mechanisms of gastric cancer multidrug resistance and future perspectives. Future Oncol. 2010;6:527–37. - PubMed
-
- Keehn RJ, Higgins GA. Chemotherapy for gastric cancer. Lancet. 1981;1(8215):323. - PubMed
MeSH terms
Substances
Grants and funding
- 3210629/FONDECYT Postdoctorado
- 3210687/FONDECYT Postdoctorado
- 1210440/FONDECYT Regular
- 1211223/FONDECYT Regular
- 15130011/FONDAP
- 20P0023/FONDEF VIU
- ID21I10027/FONDEF IDEA
- ID21I10027/FONDEF Idea
- ICN2021_045/Instituto Milenio en Inmunología e Inmunoterapia
- ICN09_016/Instituto Milenio en Inmunología e Inmunoterapia
- formerP09/016-F/Instituto Milenio en Inmunología e Inmunoterapia
- Biomedical Research Consortium-Chile/Biomedical Research Consortium-Chile
- DIUAV 03-2022/Advanced Research Grant from Universidad Autónoma de Chile
- NCS2021-013/ANID-MILENIO
- DIUA 307-2024/Research Grant from Universidad Autónoma de Chile
- IUP23-20/Interuniversity project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

