Anti-inflammatory and antioxidant properties of Camellia sinensis L. extract as a potential therapeutic for atopic dermatitis through NF-κB pathway inhibition
- PMID: 39827243
- PMCID: PMC11742993
- DOI: 10.1038/s41598-025-86678-5
Anti-inflammatory and antioxidant properties of Camellia sinensis L. extract as a potential therapeutic for atopic dermatitis through NF-κB pathway inhibition
Abstract
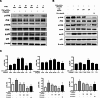
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by immune dysregulation and excessive cytokine production. This study aimed to explore the potential of Camellia sinensis L. water extract (CSE) as a treatment for AD by the impact of CSE on inflammatory responses in keratinocytes, particularly concerning the production of inflammatory cytokines and the modulation of signaling pathways relevant to AD pathogenesis. CSE was obtained via hot water extraction from Camellia sinensis L. Ultra-high-performance liquid chromatography (UPLC) analyzed catechin and caffeine content. Cell viability was assessed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), polyphenol and flavonoid content were determined. 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay measured antioxidant activity. Enzyme-Linked Immunosorbent Assay (ELISA), western blotting, and Immunofluorescence (IF) assays examined cytokines, pathways, and protein localization, respectively. Molecular docking assessed compound binding with inflammation-related proteins. UPLC identified six CSE components including epigallocatechin (EGC) epicatechin (EC), caffeine (CF), catechin (C), epigallocatechin gallate (EGCG), and epicatechin gallate (ECG). CSE demonstrated a significant reduction in the production of inflammatory cytokines interleukin (IL)-2 and IL-6 in TNF-α/IFN-γ activated keratinocytes. Treatment with CSE inhibited the mitogen-activated protein kinase (MAPK) pathway, which resulted in decreased phosphorylation of p38, Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). Exposure of TNF-α/IFN-γ- stimulated human keratinocytes (HaCaT) cells to CSE resulted in a 200 µg/mL dependent inhibition of p65 and signal transducer and activator of transcription 1 (STAT-1) translocation from the cytosol to the nucleus, as confirmed through immunofluorescence (IF) staining. Molecular docking simulations provided insights into the underlying mechanisms of CSE action, which supported its potential as a therapeutic agent for AD. CSE might be a potential candidate for its therapeutic efficacy for inflammatory skin conditions like AD. Thus, based on this evidence, the authors suggest that CSE should be studied further for its anti-inflammatory activities and topical application in the treatment of AD.
Keywords: Camellia sinensis L.; Anti-inflammation; Antioxidant; Atopic dermatitis; Ultra performance liquid chromatography.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: This study complies with all relevant institutional, national, and international guidelines and legislation.
Figures


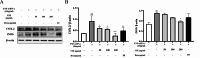





References
-
- Ryu, A. R. & Lee, M. Y. Ameliorative effect of chlorin e6-mediated photodynamic therapy on DNCB-induced atopic dermatitis-like skin lesions in mice. Mol. Cell. Toxicol.15, 265–270 (2019).
-
- Jeong, S. et al. Combined treatment of ginsenoside Rg2 and piceatannol mixture reduces the apoptosis and DNA damage induced by UVB in HaCaT cells. Mol. Cell. Toxicol.19, 63–70 (2023).
-
- Lee, K. S. et al. The prevention of TNF-alpha/IFN-gamma mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother.97, 1331–1340. 10.1016/j.biopha.2017.11.056 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

