The clinical-stage drug BTZ-043 accumulates in murine tuberculosis lesions and efficiently acts against Mycobacterium tuberculosis
- PMID: 39827265
- PMCID: PMC11742723
- DOI: 10.1038/s41467-025-56146-9
The clinical-stage drug BTZ-043 accumulates in murine tuberculosis lesions and efficiently acts against Mycobacterium tuberculosis
Abstract
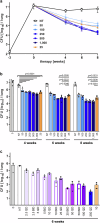
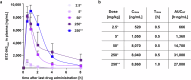
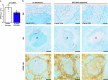
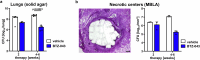
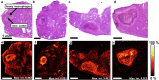
The development of granulomas with central necrosis harboring Mycobacterium tuberculosis (Mtb) is the hallmark of human tuberculosis (TB). New anti-TB therapies need to effectively penetrate the cellular and necrotic compartments of these lesions and reach sufficient concentrations to eliminate Mtb. BTZ-043 is a novel antibiotic showing good bactericidal activity in humans in a phase IIa trial. Here, we report on lesional BTZ-043 concentrations severalfold above the minimal-inhibitory-concentration and the substantial local efficacy of BTZ-043 in interleukin-13-overexpressing mice, which mimic human TB pathology of granuloma necrosis. High-resolution MALDI imaging further reveals that BTZ-043 diffuses and accumulates in the cellular compartment, and fully penetrates the necrotic center. This is the first study that visualizes an efficient penetration and accumulation of a clinical-stage TB drug in human-like centrally necrotizing granulomas and that also determines its lesional activity. Our results most likely predict a substantial bactericidal effect of BTZ-043 at these hard-to-reach sites in TB patients.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: NH has received a grant for conducting a study on delpazolid, a TB drug candidate, from LegoChem biosciences, to his institution. ELN received research funding from TB Alliance, Gates Medical Research Institute, and Janssen to his institution. MH, NH, and JD are employees of LMU Klinikum the institution that is developing BTZ-043 and have received funding from the German government. All other authors declare no competing interests.
Figures

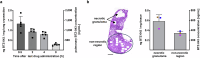
References
-
- WHO. Global tuberculosis report. (WHO, Geneva, 2023).
-
- Keshavjee, S. & Farmer, P. E. Tuberculosis, drug resistance, and the history of modern medicine. N. Engl. J. Med.367, 931–936 (2012). - PubMed
MeSH terms
Substances
Grants and funding
- TTU TB 02.810/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.814/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.806/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.902/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TI 07.003/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.705/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.710/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.709/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.813/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- TTU TB 02.901/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- SFB 1357 - 391977956/Deutsche Forschungsgemeinschaft (German Research Foundation)
- INST 91/3731-FUGG/Deutsche Forschungsgemeinschaft (German Research Foundation)
- InfectControl 03TTA01/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources
Medical

