α-Synuclein fibrils enhance HIV-1 infection of human T cells, macrophages and microglia
- PMID: 39827271
- PMCID: PMC11742913
- DOI: 10.1038/s41467-025-56099-z
α-Synuclein fibrils enhance HIV-1 infection of human T cells, macrophages and microglia
Abstract
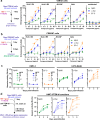
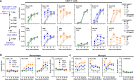
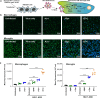
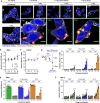
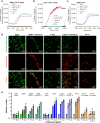
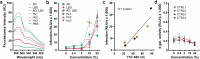
HIV-associated neurocognitive disorders (HAND) and viral reservoirs in the brain remain a significant challenge. Despite their importance, the mechanisms allowing HIV-1 entry and replication in the central nervous system (CNS) are poorly understood. Here, we show that α-synuclein and (to a lesser extent) Aβ fibrils associated with neurological diseases enhance HIV-1 entry and replication in human T cells, macrophages, and microglia. Additionally, an HIV-1 Env-derived amyloidogenic peptide accelerated amyloid formation by α-synuclein and Aβ peptides. Mechanistic studies show that α-synuclein and Aβ fibrils interact with HIV-1 particles and promote virion attachment and fusion with target cells. Despite an overall negative surface charge, these fibrils facilitate interactions between viral and cellular membranes. The enhancing effects of human brain extracts on HIV-1 infection correlated with their binding to Thioflavin T, a dye commonly used to stain amyloids. Our results suggest a detrimental interplay between HIV-1 and brain amyloids that may contribute to the development of neurodegenerative diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: DRT collaborated with Novartis Pharma AG (Basel, Switzerland), Probiodrug (Halle (Saale), Germany), GE Healthcare (Amersham, UK), and Janssen Pharmaceutical Companies (Beerse, Belgium). JM and FK are inventors on patents for using peptide nanofibrils to enhance viral transduction. Remaining authors report no competing interests.
Figures


References
-
- Nussbaum, R. L. & Ellis, C. E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 348, 1356–1364 (2003). - PubMed
-
- Hardy, J., Duff, K., Hardy, K. G., Perez-Tur, J. & Hutton, M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nat. Neurosci.1, 355–358 (1998). - PubMed
-
- Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nat. Med.10, S10–S17 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

