Chitinase-3-like-1: a multifaceted player in neuroinflammation and degenerative pathologies with therapeutic implications
- PMID: 39827337
- PMCID: PMC11742494
- DOI: 10.1186/s13024-025-00801-8
Chitinase-3-like-1: a multifaceted player in neuroinflammation and degenerative pathologies with therapeutic implications
Abstract
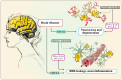
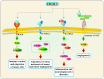
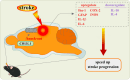
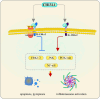
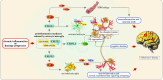
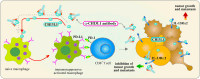
Chitinase-3-like-1 (CHI3L1) is an evolutionarily conserved protein involved in key biological processes, including tissue remodeling, angiogenesis, and neuroinflammation. It has emerged as a significant player in various neurodegenerative diseases and brain disorders. Elevated CHI3L1 levels have been observed in neurological conditions such as traumatic brain injury (TBI), Alzheimer's disease (AD), Parkinson's disease (PD), Amyotrophic lateral sclerosis (ALS), Creutzfeldt-Jakob disease (CJD), multiple sclerosis (MS), Neuromyelitis optica (NMO), HIV-associated dementia (HAD), Cerebral ischemic stroke (CIS), and brain tumors. This review explores the role of CHI3L1 in the pathogenesis of these disorders, with a focus on its contributions to neuroinflammation, immune cell infiltration, and neuronal degeneration. As a key regulator of neuroinflammation, CHI3L1 modulates microglia and astrocyte activity, driving the release of proinflammatory cytokines that exacerbate disease progression. In addition to its role in disease pathology, CHI3L1 has emerged as a promising biomarker for the diagnosis and monitoring of brain disorders. Elevated cerebrospinal fluid (CSF) levels of CHI3L1 have been linked to disease severity and cognitive decline, particularly in AD and MS, highlighting its potential for clinical diagnostics. Furthermore, therapeutic strategies targeting CHI3L1, such as small-molecule inhibitors and neutralizing antibodies, have shown promise in preclinical studies, demonstrating reduced neuroinflammation, amyloid plaque accumulation, and improved neuronal survival. Despite its therapeutic potential, challenges remain in developing selective and safe CHI3L1-targeted therapies, particularly in ensuring effective delivery across the blood-brain barrier and mitigating off-target effects. This review addresses the complexities of targeting CHI3L1, highlights its potential in precision medicine, and outlines future research directions aimed at unlocking its full therapeutic potential in treating neurodegenerative diseases and brain pathologies.
Keywords: Alzheimer’s disease; Biomarker; Brain tumors; CHI3L1; Ischemic cerebral stroke; Multiple sclerosis; Neurodegeneration; Neuroinflammation; Therapeutic targeting; Traumatic brain injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable to this review. Consent for publication: Not applicable to this review. Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this review.
Figures




References
-
- Choi J, Lee H-W, Suk K. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer’s disease. J Neurol. 2011;258:2181–5. - PubMed
-
- Wiley CA, Bissel SJ, Murdoch GH. Role of CHI3L1 in neuroinflammation. Clin Immunol (Orlando, Fla). 2015;161(2):354. - PubMed
-
- Johansen JS, Høyer PE, Larsen LA, Price PA, Møllgård K. YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem. 2007;55(12):1213–28. - PubMed
-
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53(2):172–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

