Aspergillus fumigatus is responsible for inflammation in a murine model of chronic obstructive pulmonary disease exacerbation
- PMID: 39827361
- PMCID: PMC11743040
- DOI: 10.1186/s12931-024-03092-7
Aspergillus fumigatus is responsible for inflammation in a murine model of chronic obstructive pulmonary disease exacerbation
Abstract
Background: In patients with chronic obstructive pulmonary disease (COPD), a sensitization to A. fumigatus has been related to a decline in lung function, but the role of fungal agents in the disease pathogenesis remains unclear. The main purpose of the present study was to investigate whether cell inflammation could worsen after exposure to A. fumigatus spores in vitro and then, in mice, following chronic exposure to cigarette smoke mimicking COPD.
Methods: The inflammatory response to cigarette smoke alone or with A. fumigatus was investigated in cell culture models of murine macrophages and alveolar epithelial cells. In an animal model, mice were exposed daily to two cigarettes smoke over 14 weeks, and two intranasal instillations of 105 spores at weeks 7 and 14. Then, their lungs were recovered to perform inflammatory and histopathological analyses.
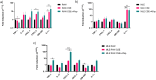
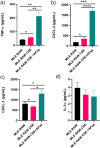
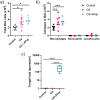
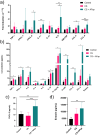
Results: In co-cultures of macrophages and epithelial cells treated with both cigarette smoke extracts (CSE) and A. fumigatus compared to CSE alone there were significant inductions in TNF-α (6.2-fold) and CXCL-2 (21.5-fold) gene expression, confirmed by significant increases in the corresponding protein secretion. In the murine model, histological analyses of the lung after chronic smoke exposure showed an increase in airspace enlargement. Moreover, a Bio-Plex approach on bronchoalveolar lavage of cigarette smoke and A. fumigatus-treated mice showed significant increases in multiple inflammatory proteins secreted in the lung.
Conclusions: There was a stronger inflammatory response after cigarette smoke exposure with A. fumigatus compared to cigarette smoke alone. These findings were correlated with histopathological changes in the mouse lung in vivo.
Keywords: Aspergillus fumigatus; Chronic obstructive pulmonary disease; Cigarette smoke; Epithelial cells; Exacerbation; Inflammation; Macrophages; Murine model.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments adhered to the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes. The protocol was approved by the French ministry of higher education and research after agreement from the local ethics committee (CECCAPP, approval number LS-2017-016). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Safiri S, Carson-Chahhoud K, Noori M, Nejadghaderi SA, Sullman MJM, Ahmadian Heris J et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679. - PMC - PubMed
-
- Barnes PJ, Burney PGJ, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primer. 2015;1(1):15076. - PubMed
-
- Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci. 2017;131(13):1541–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

