CD11c-Expressing Microglia Are Transient, Driven by Interactions With Apoptotic Cells
- PMID: 39828972
- PMCID: PMC11920677
- DOI: 10.1002/glia.24674
CD11c-Expressing Microglia Are Transient, Driven by Interactions With Apoptotic Cells
Abstract
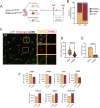
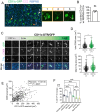
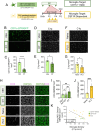
Microglia, the parenchymal macrophage of the central nervous system, serve crucial remodeling functions throughout development. Microglia are transcriptionally heterogenous, suggesting that distinct microglial states confer discrete roles. Currently, little is known about how dynamic these states are, the cues that promote them, or how they impact microglial function. In the developing retina, we previously found a significant proportion of microglia express CD11c (Integrin αX, Itgax, subunit of complement receptor 4) which has also been reported in other developmental and disease contexts. Here, we sought to understand the regulation and function of CD11c+ microglia. We found that CD11c+ microglia track with prominent waves of neuronal apoptosis in postnatal retina. Using genetic fate mapping, we provide evidence that microglia transition out of the CD11c state to return to homeostasis. We show that CD11c+ microglia have elevated lysosomal content and contribute to the clearance of apoptotic neurons, and found that acquisition of CD11c expression is partially dependent upon the TAM receptor AXL. Using selective ablation, we found CD11c+ microglia are not uniquely critical for phagocytic clearance of apoptotic cells. Together, our data suggest that CD11c+ microglia are a transient state induced by developmental apoptosis rather than a specialized subset mediating phagocytic elimination.
Keywords: CD11c; development; microglia; neuronal apoptosis; phagocytosis.
© 2025 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Update of
-
CD11c-expressing microglia are transient, driven by interactions with apoptotic cells.bioRxiv [Preprint]. 2024 Jun 26:2024.06.24.600082. doi: 10.1101/2024.06.24.600082. bioRxiv. 2024. Update in: Glia. 2025 May;73(5):1077-1089. doi: 10.1002/glia.24674. PMID: 38979153 Free PMC article. Updated. Preprint.
Similar articles
-
CD11c-expressing microglia are transient, driven by interactions with apoptotic cells.bioRxiv [Preprint]. 2024 Jun 26:2024.06.24.600082. doi: 10.1101/2024.06.24.600082. bioRxiv. 2024. Update in: Glia. 2025 May;73(5):1077-1089. doi: 10.1002/glia.24674. PMID: 38979153 Free PMC article. Updated. Preprint.
-
Lesion-remote astrocytes govern microglia-mediated white matter repair.bioRxiv [Preprint]. 2024 Mar 17:2024.03.15.585251. doi: 10.1101/2024.03.15.585251. bioRxiv. 2024. PMID: 38558977 Free PMC article. Preprint.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355
References
-
- Bejarano‐Escobar, R. , Holguin‐Arevalo M. S., Montero J. A., Francisco‐Morcillo J., and Martin‐Partido G.. 2011. “Macrophage and Microglia Ontogeny in the Mouse Visual System Can Be Traced by the Expression of Cathepsins B and D.” Developmental Dynamics 240, no. 7: 1841–1855. 10.1002/dvdy.22673. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- T32EY024234/EY/NEI NIH HHS/United States
- F31 EY035163/EY/NEI NIH HHS/United States
- S10OD026959/CA/NCI NIH HHS/United States
- S10 OD026959/OD/NIH HHS/United States
- T32NS115664/NS/NINDS NIH HHS/United States
- T32 EY024234/EY/NEI NIH HHS/United States
- R01Ey030307/EY/NEI NIH HHS/United States
- P30CA042014/NIH Office of the Director
- T32 NS115664/NS/NINDS NIH HHS/United States
- R01 EY030307/EY/NEI NIH HHS/United States
- 5P30CA042014-24/CA/NCI NIH HHS/United States
- F31EY035163/EY/NEI NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

