Guideline-directed medical therapy rates in heart failure patients with reduced ejection fraction in a diverse cohort
- PMID: 39829077
- PMCID: PMC12055396
- DOI: 10.1002/ehf2.15193
Guideline-directed medical therapy rates in heart failure patients with reduced ejection fraction in a diverse cohort
Abstract
Aims: Guideline-directed medical therapy (GDMT) is recommended for all patients with heart failure with reduced ejection fraction (HFrEF). Despite this, little data exist describing GDMT use in diverse, real-world populations including the use of vasodilators, prescribed primarily to Black populations. We sought, among a diverse population of HFrEF patients, to determine (1) GDMT use rates and target dosing by medication class and (2) predictors of GDMT use and target dosing by medication class.
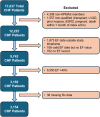
Methods: We utilized electronic health records (EHRs) from Kaiser Permanente (KP) Mid-Atlantic States, a large integrated health system. Included patients had heart failure and left ventricular ejection fraction (EF) of ≤40% between 2015 and 2021. GDMT was defined by five medication classes-angiotensin-converting enzyme (ACE) inhibitors (ACEis)/angiotensin receptor blockers (ARBs)/angiotensin receptor-neprilysin inhibitors (ARNis), beta-blockers (BBs), mineralocorticoid receptor antagonists (MRAs), sodium-glucose cotransporter 2 inhibitors (SGLT2is) and vasodilators (Black patients only). Proportions of patients on GDMT and target dose rates were examined. Logistic regression determined, within each class, predictors of medication use and being at ≥80% of the target dose.
Results: A total of 3154 patients were included. Among the 93.8% on some form of GDMT, 82.8%, 81.4%, 23.5%, 3.6% and 13.4% were on ACEis/ARBs/ARNis, BBs, MRAs, SGLT2is and vasodilators (Black patients only), respectively. Among treated patients, 45.8%, 21.4%, 77.6%, 100% and 14.7% were treated at ≥80% of the target dose for ACEis/ARBs/ARNis, BBs, MRAs, SGLT2is and vasodilators, respectively. Overall, increasing age, higher EF, atrial fibrillation/flutter, chronic obstructive pulmonary disease (COPD), prior stroke and dementia were associated with decreased odds of GDMT use. Conversely, higher body mass index (BMI), Black race, higher glomerular filtration rate (GFR), recent echo and cardiac defibrillator were associated with increased odds of GDMT use. Among treated, higher BMI, higher systolic blood pressure, haemoglobin A1C ≥ 6.5% and cardiac defibrillator were associated with higher odds of being at ≥80% of the target dose.
Conclusions: Our study using real-world data from a diverse health system demonstrated gaps in GDMT use among patients with HFrEF, specifically older patients with more comorbidities.
Keywords: guideline‐directed medical therapy; heart failure with reduced ejection fraction; medication; real‐world population; target dose.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
None declared.
Figures


References
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147‐e239. doi: 10.1016/j.jacc.2013.05.019 - DOI - PubMed
-
- Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001‐2007. doi: 10.1016/S0140-6736(99)04440-2 - DOI
-
- Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation 2002;106:2194‐2199. doi: 10.1161/01.cir.0000035653.72855.bf - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
